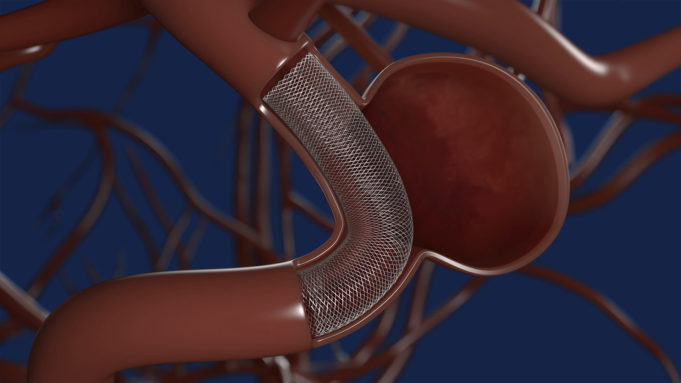
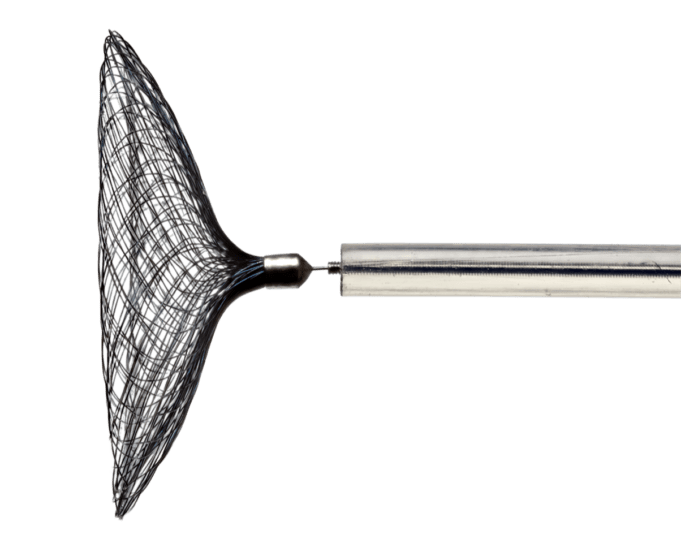
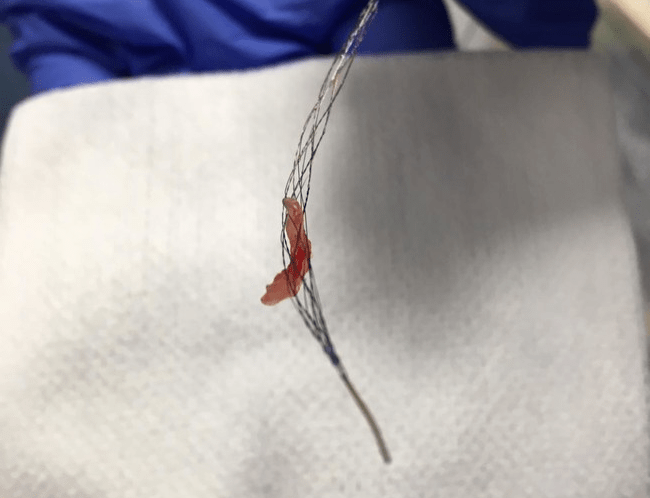
LuSeed Vascular has announced that Robert Stern, an experienced medical device executive and investor, has been nominated as the vice chairman of the company’s board of directors.
Stern currently serves as an advisor to several medical device companies and...
The Society of NeuroInterventional Surgery (SNIS) has released a position statement advocating pregnancy and parental leave policies in neurointerventional surgery, also voicing support for a physician’s ambition to have a family, as well as start, develop and maintain a...
NeuroPace has announced that the first patient with Lennox-Gastaut syndrome (LGS)—a particularly severe form of childhood-onset epilepsy characterised by cognitive dysfunction and frequent seizures—was recently treated in its feasibility investigational device exemption (IDE) study.
The study will see the company’s...
Viz.ai has announced a partnership with Cercare Medical that will incorporate the latter’s Cercare Perfusion—a fully-automated, simple-to-use, patient-specific perfusion software—into the Viz artificial intelligence (AI) platform.
In a press release, Viz.ai states that it is broadening its imaging features and...
Neurologica, a subsidiary of Samsung Electronics, has announced that its head-to-toe trauma imaging solution—the BodyTom 64 point-of-care mobile computed tomography (CT) scanner—has received 510(k) clearance from the US Food and Drug Administration (FDA) for commercial use in the USA.
Based on...
Two neurosurgeons from Tufts Medical Center in Boston, USA have been honoured with the 2022 Innovation Award at this year’s Society of Vascular and Interventional Neurology (SVIN) annual meeting (16–19 November, Los Angeles, USA) for their work on an...
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion for ‘more freedom of action in the procurement of medical products for supply of the...
The ongoing annual meeting of the Radiological Society of North America (RSNA; 27 November–1 December 2022, Chicago, USA) has seen a plethora of new research findings presented relating to various brain conditions—including attention-deficit/hyperactivity disorder (ADHD) in children, migraine-related brain...
Following the presentation of first-in-human cases with the GECKO system at the 2022 European Society of Minimally Invasive Neurological Therapy (ESMINT) congress (7–9 September, Nice, France), Basecamp Vascular (BCV) CEO and co-founder Raphaël Blanc (Foundation Rothschild Hospital, Paris, France)...
Vesalio has today announced the appointment of a new vice president (VP) of US sales, Bob Bushok, who will oversee commercial strategy and execution for the Neva VS vasospasm launch and the expected 2023 introduction of the Neva thrombectomy...
Abbott has been recognised by the Consumer Technology Association (CTA) with three Consumer Electronics Show (CES 2023; 5–8 January, Las Vegas, USA) Innovation Awards for multiple technologies that are said to be “advancing the health tech industry and improving...
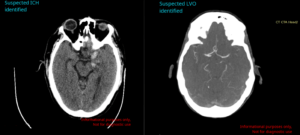
Aidoc has announced it has increased its total number of US Food and Drug Administration (FDA) clearances to 11 with the addition of two new computed tomography (CT)-based artificial intelligence (AI) solutions for detecting all intracranial vessel occlusions, and...
Viz.ai has announced a strategic partnership with full-service, vascular clinical research organisation (CRO) Vastrax. Together, the two companies intend to accelerate clinical trial enrolment for research on novel neurovascular therapies.
“Continued development of new technologies in neurointervention is the key to...
Intermittent dorsal root ganglion stimulation (DRG-S) has been shown to produce comparable results to continuous stimulation across a two-week period, as reported in Neuromodulation: Technology at the Neural Interface by investigators Kenneth Chapman (The Spine and Pain Institute of...
Mainstay Medical has announced the publication of data from a single-centre, real-world study with one-year clinical follow-up of patients selected from its ReActiv8-C study, with the results further supporting the efficacy and use of ReActiv8 restorative neurostimulation for the...
Onward Medical has been awarded a second grant from the European Innovation Council (EIC) to support the continued development of its brain-computer interface (BCI) technology for restoring mobility and upper limb function in people with spinal cord injury.
Under the...
Avicenna.AI has today announced a distribution agreement with CARPL.ai—a company providing a technology platform that enables service-provider-oriented testing, validation and deployment of artificial intelligence (AI) applications to healthcare providers.
As part of this agreement, the CARPL platform now includes Avicenna’s...
NoNO Inc has announced the dosing of the first participant in its Phase 1 clinical trial evaluating NoNO-42—a next-generation neuroprotective drug developed in the company’s PSD-95 inhibitor platform.
NoNO-42 is intended for the treatment of all people experiencing an...
The international DIRECT-SAFE trial has produced results indicating that intravenous thrombolysis (IVT) plus mechanical thrombectomy—commonly referred to as bridging therapy—should continue to be recommended as the standard treatment approach in large vessel occlusion (LVO) acute ischaemic stroke patients who...
To date, thalamic responsive neurostimulation (RNS) has shown real potential in the treatment of patients with drug-resistant epilepsy. Mark Richardson (Massachusetts General Hospital, Boston, USA) outlines why it has proven to be such a promising therapy and what the...
Cerenovus, part of Johnson & Johnson MedTech, has today announced positive primary outcomes from the real-world EXCELLENT registry, focused on stroke-inducing blood clot removal by mechanical thrombectomy, at the Society of Vascular and Interventional Neurology (SVIN) annual meeting (16–19...
At the 21st annual American Society of Regional Anesthesia (ASRA) pain medicine meeting (17–19 November 2022, Orlando, USA), SPR Therapeutics is highlighting five new data sets demonstrating significant pain relief for a majority of patients resulting from 60-day peripheral nerve stimulation (PNS)...
Route 92 Medical has announced its expansion to a new high-volume manufacturing site in Salt Lake City, USA. This 40,000-square-foot site will allow Route 92 to substantially augment its manufacturing capabilities while serving as a clinician training and education...
IRRAS recently announced it has signed a sales agency agreement to promote its IRRAflow system in the USA with Medtronic.
During the initial pilot phase of this agreement, Medtronic will have the exclusive right to promote IRRAflow in a select number of...
The urgent need for more comprehensive, standardised thrombectomy services is starting to be recognised and acted upon across Western Europe, but—as discussions at this year’s European Society of Minimally Invasive Neurological Therapy (ESMINT) congress (7–9 September, Nice, France) revealed—many countries in...
While much-needed improvements to existing triage and transport protocols for acute stroke patients were a leading topic of focus on World Stroke Day (29 October 2022), the World Stroke Congress (WSC; 26–29 October, Singapore) programme saw stroke interventions themselves...
As per its own mission statement, Collavidence wants to “make medical research collaboration and funding easier, more accessible and more transparent”, paying mind to gender and age disparities, economic barriers, and many other challenges currently facing stroke research. Mayank...
Contego Medical has announced that enrolment of the PERFORMANCE II clinical trial has been completed.
PERFORMANCE II is intended to evaluate the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) three-in-one carotid stent and post-dilation balloon system with...
CVRx—the developer of the “world’s first” US Food and Drug Administration (FDA)-approved neuromodulation device to treat the symptoms of heart failure—has launched its new Barostim NEO2 implantable pulse generator (IPG).
The second-generation device reduces the size of the IPG by...
Brainomix is set to strategically partner with Visionable, a real-time clinical collaboration platform designed to enable virtual healthcare models, in an attempt to transform the delivery of stroke care.
The two tech-enabled health companies will collaborate and comarket two complementary...
Viz.ai has shared highlights from new data set to be presented at next week’s Society of Vascular and Interventional Neuroradiology (SVIN) annual meeting (16–19 November 2022, Los Angeles, USA) demonstrating the significant impact of Viz ANEURYSM at a large...
In a new paper published in Light: Science and Applications, a team of scientists and engineers led by Ji-Xin Cheng and Chen Yang (Boston University, Boston, USA) has developed an optically generated focused ultrasound (OFUS) method for non-invasive brain...
RapidAI has announced receipt of US Food and Drug Administration (FDA) 510(k) clearance for the latest release of Rapid ICH—a technology that it describes as “the best-performing” intracranial haemorrhage (ICH) triage and notification product on the market, with a sensitivity...
The Wyss Center for Bio and Neuroengineering in Geneva, Switzerland has today revealed the latest preclinical neural data acquired with its fully implantable ABILITY brain-computer interface (BCI) system. The results, to be presented at the Society for Neuroscience meeting...
Insightec has announced the publication of what it claims is the largest prospective, long-term follow-up study to date of unilateral magnetic resonance imaging-guided focused ultrasound (MRgFUS) thalamotomy for essential tremor, demonstrating sustained and significant tremor improvements that were maintained...
Researchers led by a vascular team in Indianapolis found that carotid endarterectomy (CEA) remains the most-used strategy in carotid revascularisation, with CEA and transcarotid artery revascularisation (TCAR) showing decreased odds of stroke or death compared to transfemoral carotid artery...
A common theme surrounding last month's World Stroke Day (29 October) was the fact that, while acute ischaemic stroke therapies may have advanced markedly over the past few years, there is still much room for improvement. Researchers are not...
As part of its Medicare Telehealth Services List, the US Centers for Medicare and Medicaid Services (CMS) will continue to cover the use of Abbott’s NeuroSphere virtual clinic for programming deep brain stimulation (DBS) devices beyond the COVID-19 public...
Based on his leading experience with the hydrophilic polymer coating (HPC)-coated pEGASUS stent (phenox) at his centre, and positive, early observations of this easy-to-use device, André Kemmling (University Medical Center Marburg, Marburg, Germany) details the benefits of surface modification in treating...
Vascular device company iVascular has announced the expansion of its therapeutic areas with the launch of a new neurovascular pipeline, having gained CE-mark approval for four devices in this space: the iNdeep microcatheter, iNedit balloon distal access catheter, iNtercept...
Results from the BAOCHE randomised controlled trial (RCT), which found that the addition of thrombectomy to standard medical care within 6–24 hours in basilar artery occlusion stroke patients improved functional outcomes at 90 days—as compared to standard care alone—have...
NOTE: This video is ONLY available to watch in selected countries and geographies
Given the array of potential benefits associated with robotic-assisted techniques in the neurointervention space—including improved precision and control, as well as reduced radiation exposure and infection...
Medtronic has announced the first results from its DiVERT clinical study, which showed that post-stroke workflow protocols and cardiac monitoring vary significantly across community hospitals and academic centres. These data were presented at the 14th annual World Stroke Congress...
On World Stroke Day (29 October 2022), the World Stroke Organization (WSO) has announced the launch of a new programme that aims to drive access to quality acute stroke care and save lives around the world.
The WSO Stroke Certification...
On World Stroke Day 2022 (29 October), US physicians from the Get Ahead of Stroke campaign are urging the public to embrace the “simple truth” that strokes are emergencies—and they require calling emergency medical services (EMS) for immediate triage,...
Synchron has announced that enrolment in the COMMAND trial has commenced at the University of Pittsburgh in Pittsburgh, USA.
The COMMAND trial is an early feasibility study funded by the National Institutes of Health (NIH) that will primarily assess safety...
Medtronic has announced the launch of its neurovascular Co-Lab platform, which is designed to accelerate "urgently needed" innovation in stroke care and treatment.
The company is harnessing its market-leading position and trusted partner reputation to create a platform that will...
NICO Corporation has completed a US$12.5 million capital raise in an oversubscribed round that will scale the business, and leverage continued progress on the company's recently completed adaptive clinical trial for intracerebral haemorrhage (ICH) and a new partnership in functional precision oncology for patients with deadly,...
NeuroOne Medical Technologies has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market its Evo stereoelectroencephalography (sEEG) electrode technology for temporary (less than 30 days) use with recording, monitoring and stimulation equipment for the...
Bruce Ovbiagele, associate dean and professor of neurology at the University of California San Francisco (UCSF; San Francisco, USA) and chief of staff at the San Francisco Veteran Affairs Health Care System, has been named the new editor-in-chief of...
Royal Philips today announced it will showcase the company’s latest advances to support the treatment of stroke patients at the 14th annual World Stroke Congress (WSC 2022, 26–29 October, Singapore).
As per a company press release, Philips is working...
Following the publication of a case report in Topics in Magnetic Resonance Imaging, researchers from Malaysia have indicated that the use of vessel-wall imaging (VWI) “could potentially serve as an imaging method to presumptively diagnose spontaneous recanalisation in patients...
Neurotechnology start-up Axoft has launched and announced US Food and Drug Administration (FDA) Breakthrough Device designation for its brain-machine interface (BMI) technology intended to better the treatment of neurological disorders.
The company secured US$8 million in capital to fund...
Earlier this year, results from the Canadian AcT (Alteplase compared to tenecteplase) randomised controlled trial (RCT) were delivered for the first time at the European Stroke Organisation Conference (ESOC 2022; 4–6 May, Lyon, France). And, alongside other first-time data...
Theranica Bio-electronics has announced positive top-line results in its double-blind, randomised, placebo-controlled, multicentre, pivotal clinical trial in the USA. This trial evaluated the efficacy and safety of Nerivio—a wearable remote electrical neuromodulation (REN) device—for the preventive treatment of migraine...
RAPID Aneurysm, a semi-automated artificial intelligence (AI) software programme (RapidAI), is highly accurate in detecting cerebral aneurysms on computed tomography angiography (CTA) imaging, according to a retrospective study published recently in the Journal of Stroke and Cerebrovascular Diseases by...
Vesalio has announced EU Medical Device Regulation (MDR) certification of its product lines—achieving this “significantly ahead” of its May 2023 deadline, according to a company press release.
This milestone is the third in a series of recent regulatory approvals of...
Deep brain stimulation (DBS) can halve the symptoms of severe obsessive-compulsive disorder (OCD), according to the findings of a pooled data analysis of available evidence, which has been published online in the Journal of Neurology, Neurosurgery and Psychiatry (JNNP). The...
A new white paper, released by the Wyss Center for Bio and Neuroengineering (Geneva, Switzerland), reveals the first neural signals recorded by the ABILITY brain-computer interface (BCI) system. It also lays out the plan for a forthcoming human clinical...
Nevro Corporation has announced that it has received approval from the US Food and Drug Administration (FDA) for the Senza HFX iQ spinal cord stimulation (SCS) system.
The company will initiate a limited release of Senza HFX iQ in the USA this...
The Society of Vascular and Interventional Neurology (SVIN) has said it wants to create awareness around stroke events leading up to World Stroke Day on 29 October, and will do so by challenging people to have #NoMercyOnStroke.
The challenge to have #NoMercyOnStroke...
Five-year outcomes of a multicentre, randomised controlled trial (RCT) indicate that carotid endarterectomy (CEA) or carotid artery stenting (CAS) are not superior to best medical therapy (BMT) alone for moderate-to-severe asymptomatic carotid artery stenosis.
Tilman Reiff (University Hospital of...
NeuroPace has announced the first patient has been implanted in the NAUTILUS clinical trial, a pivotal study that will evaluate the safety and effectiveness of its RNS (restorative neurostimulation) system in individuals aged 12 and older with drug-resistant idiopathic...
Neuspera Medical has announced the first patient has been successfully implanted with the Nuvella system in its pivotal SANS-UUI clinical trial, which will evaluate the safety and efficacy of the system—designed to treat overactive bladder (OAB) with sacral neuromodulation...
IRRAS today announced the launch of a new educational webinar series, entitled “Exploring the Impact of IRRAflow's Active Fluid Exchange: Our Early Experience Utilizing IRRAflow for Intracranial Hemorrhage”, alongside the Jacobs Institute (Buffalo, USA).
During each live webinar, Adnan Siddiqui (University...
A new report from a joint taskforce of the American College of Radiology (ACR) and the Society of Interventional Radiology (SIR) recommends improved access to interventional radiologists in small and rural areas. The taskforce was formed to explore strategies for...
Researchers in the USA have developed an injectable, ‘toothpaste-like’ biomaterial that may offer promise in the treatment of intracranial aneurysms as an alternative to endovascular coil placement, surgical clipping procedures or injected liquid blocking agents—all of which carry possible...
Phagenesis today announced that the US Food and Drug Administration (FDA) has granted a de novo request for the Phagenyx neurostimulation system, which uses pharyngeal electrical stimulation (PES) to restore swallowing control in patients with severe dysphagia post stroke....
Technical and clinical outcomes associated with mechanical thrombectomy treatments in England are currently “acceptable”, but ‘drip-and-ship’ patients, process times and variation between units all represent concerns in stroke care moving forward, as per discussions at this year’s European Society...
NICO Corporation has announced that enrolment is now complete for the ENRICH (Early minimally invasive removal of ICH) randomised controlled trial, which is comparing medical and economic outcomes between early surgical intervention for intracerebral haemorrhage (ICH) with minimally invasive parafascicular surgery (MIPS) to current guideline-directed medical...
Achieving clot retrieval with the fewest possible number of passes is critical in mechanical thrombectomy, and may even hold greater importance than how quickly the procedure is completed—despite the age-old mantra that ‘time is brain’. That is according to...
Nevro Corporation announced recently that the US Food and Drug Administration (FDA) has approved its manufacturing operations in Costa Rica for the production of its proprietary spinal cord stimulation (SCS) systems in the treatment of chronic pain—including its HFX product platform.
This...
Tenecteplase, a newer-generation intravenous thrombolysis (IVT) drug, has outperformed the traditional treatment (alteplase) for ischaemic strokes in several key areas, demonstrating better health outcomes and lower costs, according to a recent study published in the journal Stroke.
The study was led by a...
The latest and greatest in neurointerventional clinical research buzzed our readers in September, with new studies on dual antiplatelet therapy (DAPT) duration, radial versus femoral access approaches, and a possible shift towards endovascular aneurysm treatments, all garnering plenty of...
Microvention, a wholly owned subsidiary of Terumo Corporation, today celebrated its 25-year anniversary in the neuroendovascular technologies industry.
The capstone of Microvention’s 25th anniversary celebration will include ‘Patient's Day’. Timothy White, a neurosurgery chief resident and endovascular fellow at the...
Wallaby/phenox has officially announced the successful global launch of its novel Pegasus hydrophilic polymer coating (HPC) stent, having first unveiled the device at the 2022 European Society of Minimally Invasive Neurological Therapy (ESMINT) congress (7–9 September, Nice, France).
The introduction of the...
In the randomised HD-RECOVERY clinical trial, the use of high-definition transcranial direct current stimulation (HD-tDCS) has been found to be associated with a statistically significant increase in the number of ventilator-free days among critically ill COVID-19 patients.
Writing in the...
Magnus Medical has announced receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for its SAINT neuromodulation system for the treatment of major depressive disorder (MDD) in adults who have failed to achieve satisfactory improvement from prior...
This advertorial is sponsored by phenox.
Following the publication of a study assessing the use of Hydrophilic Polymer Coating (HPC) technology with the p64 MW flow modulation device (phenox) at her centre earlier this year, Victoria Hellstern (Klinikum Stuttgart, Stuttgart,...
Mainstay Medical has announced that the National Institute for Health and Care Excellence (NICE), a public body of the UK Department of Health, has issued a recommendation that the company’s ReActiv8 restorative neurostimulation therapy can be used in the...
A recent sub-analysis of the SWIFT-DIRECT trial, which is published in the Journal of NeuroInterventional Surgery (JNIS), has found “no evidence” that the effect of bridging intravenous thrombolysis (IVT) on functional independence outcomes is modified by overall or in-hospital...
The European Society for Vascular Surgery (ESVS) has released clinical practice guidelines on radiation safety, which the writing committee notes are the first guidelines on the topic to be published under the auspices of a vascular surgical society. The...
Theranica has announced the publication of a new study comparing the effectiveness of Remote electrical neuromodulation (REN) to that of standard-care medications for the acute treatment of chronic migraine. The post-hoc analysis—published in Pain Management—concludes that REN administered by Theranica's flagship...
Among patients undergoing transfemoral transcatheter aortic valve implantation (TAVI), the use of a cerebral embolic protection device (Sentinel, Boston Scientific) did not have a significant effect on the incidence of periprocedural stroke.
However, a difference in rates of disabling stroke,...
Vesalio has announced US Food and Drug Administration (FDA) humanitarian device exemption (HDE) approval for commercialisation of its NeVa VS device, which is indicated for the adjunct treatment of symptomatic cerebral vasospasm following aneurysmal subarachnoid haemorrhage (aSAH).
According to a...
The duration of dual antiplatelet therapy (DAPT), either 30–90 days or more than 90 days, following implantation of a flow diverter in cerebral aneurysm patients has been found to have no significant effect on major complications and adverse events....
Aleva Neurotherapeutics has announced CE-mark approval of its magnetic resonance imaging (MRI) labelling for the directSTIM deep brain stimulation (DBS) system, allowing the technology to be used in a full-body MRI environment across Europe.
The directSTIM system, featuring a fully...
Sheba Medical Center, which is located in Ramat Gan, Israel and claims to be the country’s largest medical centre, has announced a major collaboration agreement with Thomas Jefferson University (Philadelphia, USA) to advance neuroscience research and clinical care across...
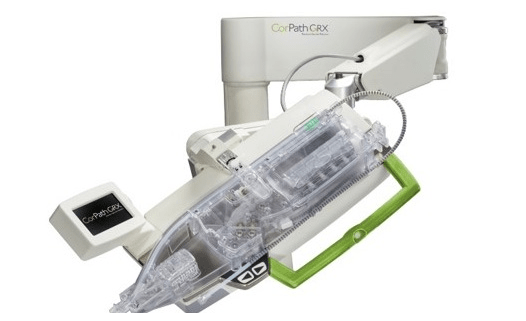
This advertorial, sponsored by Siemens Healthineers, is intended for readers outside of the USA.
The majority of cases the CorPath GRX Neurovascular Robotic System (Siemens Healthineers) has been deployed in to date are cerebral aneurysm treatments— primarily neurovascular coiling and...
The Neqstent coil-assisted flow diverter (Cerus Endovascular) has demonstrated comparable occlusion rates to those seen with other, similar flow diversion technologies, as well as positive device stability and safety outcomes. These new data were presented by Thomas Liebig (Ludwig...
Onward Medical recently announced that the Up-LIFT pivotal study evaluating its non-invasive spinal cord stimulation (SCS) ARC-EX therapy achieved its primary effectiveness endpoint of improvement in upper extremity strength and function in patients with movement disabilities.
“Restoring hand and arm...
Corindus, a Siemens Healthineers company, announced today the recent presentation of results from a “first-of-its-kind” study demonstrating the safety and effectiveness of robotic-assisted neurovascular aneurysm embolisation using the CorPath GRX system. The data represent a milestone in the company’s...
A global study published in the Journal of Neurology, Neurosurgery and Psychiatry (JNNP) has revealed that, while ruptured aneurysm clipping rates remained largely unchanged, there was an increased rate of ruptured aneurysm coiling between 2019 and 2021—which the authors...
Robert Hamilton (Los Angeles, USA) outlines several key milestones in the history of stroke care, and discusses how recent technological advances in this space will likely benefit physicians and their patients in the years to come.
Throughout the world, technology...
Axonics has announced the first patient implants in Canada with Axonics F15, the company’s newly developed, long-lived, fully recharge-free sacral neuromodulation (SNM) system.
The University of Alberta (Edmonton, Canada) implanted four patients this week with the Axonics F15, according to...
Basecamp Vascular (BCV) has announced a “major step” in the development and evaluation of steerable mechatronic guidewires for stroke treatment via its GECKO system.
A first-in-human case with the device was conducted by Michel Piotin (Foundation Rothschild Hospital, Paris,...
Silk Road Medical has announced enrolment of the first patient in ROADSTER 3, which the company claims is the the first prospective, multicentre, single-arm study to assess real-world treatment of standard surgical risk patients with carotid artery disease using...
Home-based, self-administered transcranial direct current stimulation (tDCS) has demonstrated feasibility, acceptability and significant reductions in clinical pain intensity in older adults with knee osteoarthritis, as per a randomised controlled trial (RCT) published in the journal Brain Stimulation.
The study’s authors,...
Penumbra announced today that its RED reperfusion catheters have secured a CE mark and are now commercially available in Europe. The catheters are part of the company’s Penumbra system, which is a fully integrated mechanical aspiration thrombectomy system designed to...
Viz.ai has received US Food and Drug Administration (FDA) 510(k) clearance for an automated right ventricle-to-left ventricle (RV/LV) ratio algorithm, a new component of its Viz PE Solution.
The algorithm is designed to quickly and accurately measure the diameter of...
Isil Saatci has been practising interventional neuroradiology (INR) in Ankara, Turkey for almost the entirety of her career—initially at Hacettepe University, where she also attended medical school, and currently at Koru Hospitals. Saatci was also the first woman to...
Nevro Corporation has announced that US health plan Aetna has updated its spinal cord stimulation (SCS) coverage policy to explicitly cover painful diabetic neuropathy (PDN), effective from 29 August 2022.
Nevro also recently announced that Novitas and First Coast, the...
Insightec has announced that it has signed a credit facility for up to US$200 million with affiliates of existing shareholders, Perceptive Advisors and Community Fund. The financing, according to a press release, has provided Insightec with US$100 million at closing, with an...
In August, NeuroNews’ most read stories included new clinical data on a number of neurovascular technologies, physician viewpoints on a “historic” neurointerventional procedure in the USA, an update from the Women in Neuromodulation group, and more.
1. J Mocco becomes...
Neurofenix has raised US$7 million in Series A funding via a round led by AlbionVC, with additional participation from HTH, InHealth Ventures, and existing investors, according to a company press release.
The release notes that this will enable Neurofenix to...
Brainomix has announced that it has been awarded the national tender in Hungary to deploy its artificial intelligence (AI) stroke imaging software across all stroke centres in the country as part of a National Institute for Health Development initiative to improve stroke care.
Awarded...
Vesalio has today announced receipt of CE-mark approval for commercialisation of its new NeVa NET, which the company claims is the “first and only” thrombectomy device with an integrated clot microfiltration technology.
This unique stent retriever combines Vesalio’s proprietary Drop...
Gimer Medical today announced that the company’s spinal cord stimulation (SCS) system was granted conditional investigational device exemption (IDE) approval by US Food and Drug Administration (FDA) on 13 August.
According to a company press release, Gimer is now...
Highlights:
Details and comment on the first US implantation of the Stentrode brain-computer interface device (Synchron) lead a number of late-breaking presentations from this year’s Society of NeuroInterventional Society (SNIS) annual meeting
The Stroke Association calls for a 24/7...
Highlights:
Details and comment on the first US implantation of the Stentrode brain-computer interface device (Synchron) lead a number of late-breaking presentations from this year's Society of NeuroInterventional Society (SNIS) annual meeting
The Stroke Association calls for a 24/7...
After a long period of uncertainty triggered by a worldwide pandemic, the 2022 European Society of Minimally Invasive Neurological Therapy (ESMINT) congress (7–9 September, Nice, France) will bring back its in-person offering and become a hybrid event next week....
A prospective analysis of 200 consecutive diagnostic cerebral angiograms has indicated that the number of diffusion-weighted magnetic resonance imaging (DW-MRI) restriction foci was “significantly more frequent” when transradial access was used—as compared to the more established transfemoral access approach.
As...
Theranica has announced the closing of its Series C funding round, with a first closing of US$45 million. New Rhein Healthcare Investors led the round, with wide participation from existing investors aMoon, Lightspeed Venture Partners, LionBird, Takoa Invest and...
CereVasc today announced completion of the first eShunt procedure in the USA as part of a pilot study involving normal-pressure hydrocephalus (NPH) patients.
The study is being conducted under an investigational device exemption (IDE) approved by the US Food and Drug Administration (FDA)...
MicroPort Neurotech today announced that it has received marketing approval issued by the Ministry of Health, Labour and Welfare of Japan for its independently developed Numen coil embolisation system—marking the first approved product in Japan for the company.
Numen is...
AiM Medical Robotics has announced the unveiling of a magnetic resonance imaging (MRI)-compatible prototype of its “industry-first” robotics platform, a company press release states, as well as the receipt of funding from the Sontag Foundation’s Sontag Innovation Fund.
"The prototype...
Following the first endovascular implantation of Synchron’s Stentrode brain-computer interface (BCI) device in the USA, physicians have highlighted the wider significance of this breakthrough case—not only in progressing a technology that stands to fulfil an unmet need for patients...
Penumbra today announced initial INSIGHT registry data showing that its RED reperfusion catheters were successful in removing all clot types—and, additionally, results of sub-analyses from the company’s COMPLETE study indicated the Penumbra system’s effectiveness for acute ischaemic stroke patients...
Imperative Care recently announced that new data evaluating the utility of its Zoom stroke solution were presented at the 16th congress of the World Federation of Interventional and Therapeutic Neuroradiology (WFITN 2022; 21–25 August, Kyoto, Japan).
Results from an independent,...
IRRAS has announced the initial patient treatments with the company's IRRAflow system within Mount Sinai Health System (New York, USA).
Thus far, two patients suffering from intraventricular haemorrhage (IVH) have been enrolled in the DIVE (Deployment of irrigating intraventricular catheter system) clinical...
Abbott has announced that the US Food and Drug Administration (FDA) has approved its new Proclaim Plus spinal cord stimulation (SCS) system featuring FlexBurst360 therapy. The company describes FlexBurst360 as the next generation of its proprietary BurstDR stimulation, offering...
Ybrain recently received the Translational Research Award at this year's Neuroergonomics and NYC Neuromodulation Conferences (28 July–1 August, New York City, USA) for its transcranial direct current stimulation (tDCS) technology.
This recognition was conferred to researchers who made a significant contribution to...
J Mocco, an endovascular neurosurgeon at Mount Sinai in New York City, USA has been named the 2022 president of the Society of NeuroInterventional Surgery (SNIS) and will lead the SNIS’ initiatives to advance excellence in minimally invasive neuroendovascular...
In light of recent progress made in alleviating gender disparities within the neuromodulation field, Magdalena Anitescu (Chicago, USA), Anne Fennimore (Boca Raton, USA), Kiran Patel (New York, USA) and Julie Pilitsis (Boca Raton, USA) of the Women in Neuromodulation...
Motion analysis of video recorded on a smartphone accurately detected narrowed carotid arteries, according to new research published in the Journal of the American Heart Association.
"Between 2% and 5% of strokes each year occur in people with no symptoms, so...
Minnetronix Medical’s first product, which the company says was conceptualised, developed, US Food and Drug Administration (FDA)-cleared and manufactured internally, is now fully commercialised and available to the neurosurgery market.
The only expandable brain access port on the market—according to...
Data from a randomised controlled trial (RCT), which have been published in Frontiers in Neurology, support the use of neuromodulation with transcranial direct current stimulation (tDCS) as an add-on to neurorehabilitation in the treatment of Parkinson’s disease patients affected...
Vena Medical has announced the successful treatment of the first five patients in the world using its Vena Balloon Distal Access Catheter (BDAC) at London Health Sciences Centre University Hospital (London, Canada) and The Ottawa Hospital (Ottawa, Canada).
The Health...
The Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July 2022, Toronto, Canada) saw research involving the Woven EndoBridge (WEB; Microvention/Terumo) device disclosed for the first time, with co-principal investigator Adam Arthur (University of Tennessee Health Sciences Center and Semmes-Murphey...
A multicentre study of more than 3,000 people with high blood pressure and brain aneurysms found that the use of renin-angiotensin-aldosterone system (RAAS) inhibitors—a class of blood pressure-lowering medications—reduced the risk of an aneurysm rupture by 18%. These findings...
SyncThink announced today that it has gained CE-mark certification for its EYE-SYNC technology, affirming conformity to the European Medical Device Regulation (MDR). EYE-SYNC will be registered as a Class I medical device, according to a company press release.
“The CE-mark...
Research from Ochsner Health (New Orleans, USA) published in the Journal of the American College of Cardiology (JACC) is likely to influence expanded insurance coverage for carotid artery stenting.
The paper, "Carotid artery stenting: JACC state-of-the art review", details significant advances...
NeuroOne Medical Technologies has announced the submission of a special 510(k) to the US Food and Drug Administration (FDA) for its stereoelectroencephalography (sEEG) electrode to extend the duration of use from less than 24 hours to less than 30...
CereVasc announced today that the US Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application to initiate a pilot trial of the eShunt system in patients who develop communicating hydrocephalus post-aneurysmal subarachnoid haemorrhage (paSAH).
"Considering the encouraging...
NaoTrac, a CE-certified neurosurgical navigation robot from Brain Navi Biotechnology, has achieved approval from Taiwan's Food and Drug Administration (TFDA), and submission to the US FDA for clearance by the end of 2022 is now planned.
The NaoTrac human trials...
The National Institutes of Health (NIH) Common Fund has named eight winners of the first phase of the Neuromod Prize—a US$9.8 million competition to accelerate the development of targeted neuromodulation therapies that adjust nerve activity to improve organ function.
The...
Updates from two major neurointervention events, the Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July 2022, Toronto, Canada) and LINNC Paris (30 May–1 June 2022, Paris, France), drew plenty of attention throughout July—as did key updates relating to...
Following a retrospective review of databases from multiple different centres, researchers have reported the safe and effective use of the Silk Vista Baby (Balt) flow diversion device in the treatment of complex posterior inferior cerebellar artery (PICA) aneurysms.
Writing in...
Abbott recently announced the Health Canada licensing of NeuroSphere virtual clinic, a remote programming technology that the company claims is the first of its kind in Canada and is compatible with Abbott's suite of neuromodulation technologies.
NeuroSphere virtual clinic has the potential to...
Late-breaking research presented at the recent Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada) has shown that thrombectomy procedures can achieve positive outcomes for patients experiencing strokes caused by a basilar artery occlusion (BAO)—despite typically...
A new report released by the UK Stroke Association warns that, if thrombectomy rates remain at 2020/21 levels, a total of 47,112 stroke patients in England would miss out on “game-changing” mechanical thrombectomy treatments over the length of the...
Stroke patients with COVID-19 are facing worse outcomes, and are often younger and healthier, according to research presented recently at the Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada). People with COVID-19 are more than...
A new study presented at the Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada) shows that artificial intelligence (AI) technology can identify when a patient is having a stroke caused by emergent large vessel occlusion...
Implementing severity-based field triage leads to faster treatment and less disability for stroke patients, according to late-breaking research presented recently at the Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada).
These findings, which are now also...
Stroke surgery and other neuroendovascular procedures could be made safer and easier through robotics, according to research presented at the Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada).
A new multi-articulated, self-steering microcatheter for neuroendovascular...
Imperative Care has announced that new data from studies evaluating the utility of its Zoom stroke solution were recently presented at the ongoing Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada).
Results from an independent, single-centre...
The American Heart Association (AHA) has announced that Mitchell Elkind (Columbia University, New York City, USA), long-time volunteer of the organisation and renowned neurologist, will be joining the staff leadership team later this year as chief clinical science officer....
Viz.ai today announced it has received US Food and Drug Administration (FDA) 510(k) clearance for Viz Subdural (SDH). The Viz SDH algorithm uses artificial intelligence (AI) to automatically detect subdural haemorrhage, enabling physicians to triage patients effectively and deliver...
Rapid Medical announced at the ongoing Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada) that it has recently received US Food and Drug Administration (FDA) 510(k) clearance for its TIGERTRIEVER 13 device to treat large...
Route 92 Medical recently announced the publication of an investigator-initiated, multicentre SLIC (Super large-bore ingestion of clot) study of its proprietary Monopoint operating platform, showing 82% first-pass efficacy, according to a press release.
Results from 33 consecutive patients also...
RapidAI has announced receipt of US Food and Drug Administration (FDA) clearance for Rapid Hyperdensity—the newest addition to the RapidAI platform. According to the company, this tool empowers physicians to quickly assess the severity of injury in patients with acute...
An “interesting case” of unilateral proptosis in a 14-year-old girl, treated using a hybrid approach via direct external carotid artery (ECA) puncture, was presented during a roundtable session at this year’s LINNC Paris Course (30 May–1 June, Paris, France)...
The SPINNERS study, which has just been announced by Siemens Healthineers, will evaluate the diagnostic accuracy of non-contrast flat-detector computed tomography (CT) compared to multi-slice CT for detecting intracranial haemorrhage in stroke patients.
The recently registered study is an investigator-initiated...
Specialists from around the world will gather at the Society of NeuroInterventional Surgery's (SNIS) 19th annual meeting (25–29 July, Toronto, Canada) next week to discuss groundbreaking research and developments in the field of neurointerventional surgery.
The 2022 conference, held during SNIS'...
Cerenovus, part of Johnson & Johnson MedTech, will showcase its latest innovations in stroke care at the 2022 Society of NeuroInterventional Surgery (SNIS) annual meeting (25–29 July, Toronto, Canada). The company is set to announce new clinical data during...
Synchron has announced the first human brain-computer interface (BCI) implant in the USA. This procedure represents a significant technological milestone for scalable BCI devices and is the first to occur in the USA using an endovascular BCI approach, which...
Saluda Medical has announced that Steven Falowski (Argires-Marotti Neurosurgical Associates of Lancaster, Lancaster, USA) recently presented late-breaking 24-month data from the company’s EVOKE study at the 2022 American Society of Pain and Neuroscience (ASPN) annual meeting (14–17 July, Miami Beach,...
Imperative Care has announced that new data from studies evaluating the utility of the Zoom stroke solution for the treatment of ischaemic stroke will be presented at the Society of NeuroInterventional Surgery (SNIS)’s 19th annual meeting (25–29 July; Toronto,...
In-depth discussions regarding a “landmark” study in medium vessel occlusion (MeVO) stroke, and the first thrombectomy procedures with a novel balloon guide catheter device, were among the most read NeuroNews stories in June. An exclusive interview with a pioneering...
Among people who received endovascular mechanical thrombectomy to remove a large vessel occlusion (LVO), those with a rare genetic disease variant found primarily in people of East Asian descent were more likely to have the vessel reclog during, or shortly...
The world of interventional neuroradiology will likely need to follow the cardiology field and conduct large-scale prospective studies in order to move away from ‘eminence-based medicine’, and gain a better understanding of antithrombotic medication outcomes. “I think we can...
Abbott recently announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation to investigate the use of its deep brain stimulation (DBS) system in treatment-resistant depression (TRD).
As per a company press release, Abbott's DBS system...
NICO Corporation recently announced that it has partnered with Imedcare, an Australia-based supplier and distributor of devices, as its distributor for the neurosurgery markets in the medical sectors of Australia and New Zealand.
"We look forward to expanding access to NICO's innovative technologies in this...
NeuroOne Medical Technologies today announced that the first clinical case using the Evo stereoelectroencephalography (sEEG) electrode was recently performed by Robert Gross at Emory University in Atlanta, USA.
Gross selected the Evo sEEG electrode for intraoperative brain mapping at the subsurface...
Nevro Corporation has announced that the complete 12-month results from the SENZA-PDN randomised controlled trial (RCT), including health-related quality of life outcomes in patients with painful diabetic neuropathy (PDN) treated with high-frequency 10kHz spinal cord stimulation (SCS), have been published...
Neuromod Devices has published the results of the company's TENT-A2 (Treatment evaluation of neuromodulation for tinnitus—stage A2) clinical trial in Scientific Reports via a paper titled “Different bimodal neuromodulation settings reduce tinnitus symptoms in a large randomized trial”.
The TENT-A2...
NeuroVasc Technologies recently announced that the first patient has been treated in its US investigational device exemption (IDE) clinical study, the ENVI randomised controlled trial (RCT). The ENVI RCT will evaluate the safety and efficacy of the company’s ENVI-SR...
A systematic review and meta-analysis published in Nature Schizophrenia has concluded that data from sham-controlled studies to date indicate the effectiveness of transcranial magnetic stimulation (TMS) in treating the negative symptoms of schizophrenia. In addition, this analysis’ authors—Søren Østergaard...
A preliminary report lends evidence to support female sex being considered as an independent risk factor for new stroke occurrence after carotid revascularisation in symptomatic patients, according to a research team from Italy who presented at the International Chapter...
Electrical stimulation technologies, and in particular spinal cord stimulation (SCS) and sphenopalatine ganglion stimulation, offer a promising adjunct to existing therapies for cerebral vasospasm after subarachnoid haemorrhage—as per a recent, systematic literature review.
Writing in Neuromodulation: Technology at the...
The Clotild smart guidewire system (Sensome), which uses artificial intelligence (AI) to provide real-time insights on clot composition, is showing early promise in the first-in-human CLOT OUT study, according to Hal Rice (Gold Coast University Hospital, Southport, Australia). Rice...
In what is claimed to be the largest stroke clinical trial ever run in Canada, researchers have shown that tenecteplase—a safe, well-tolerated drug commonly used as a clot-buster for heart attacks—is an effective treatment for acute ischaemic stroke. They...
Over the course of the past two years, the accepted understanding of how COVID-19 spreads and functions has improved vastly, with preventative vaccines and therapeutic drugs enabling patients to be treated more effectively. However, the relationship between the virus...
Adham Elmously (New York Presbyterian Hospital, New York, USA) presented outcomes of early transcarotid artery revascularisation (TCAR) versus carotid endarterectomy (CEA) after acute neurologic events during the opening day of the Vascular Annual Meeting (VAM; 15–18 June, Boston, USA).
Speaking...
In one of the first studies to investigate the overall quality of care and outcomes of stroke care for non-COVID-19 hospitalised patients during the pandemic from a US perspective, researchers have reported no decline in overall quality of care...
Neuspera Medical recently announced it will begin enrolment in its pivotal clinical trial, SANS-UUI—a single-arm investigational device exemption (IDE) study that will enrol 145 patients at 25 sites globally.
The study will evaluate the safety and efficacy of Neuspera's Nuvella system,...
Valencia Technologies Corporation has announced in a press release that it has completed the “world's first” commercial implantation of its eCoin leadless tibial neurostimulator, which gained US Food and Drug Administration (FDA) approval as an urge urinary incontinence treatment...
The results of an analysis of nearly 2,000 carotid endarterectomies (CEAs) “challenge the notion” that patients benefit significantly from the current postoperative surveillance guidelines, and suggest that these may be contributing to “oversurveillance”—an issue that should be addressed in...
Stimwave Technologies Incorporated (Stimwave) recently announced that it has reached an agreement to sell substantially all of its assets to Kennedy Lewis Management LP (Kennedy Lewis).
Since 2020, the company has successfully re-structured its entire team and business operations—according to...
MicroTransponder has closed an oversubscribed US$53 million Series E funding round led by US Venture Partners (USVP), a multi-stage investment firm. GPG Ventures and Exceller Hunt Ventures return as existing investors alongside new venture investors that include Osage University...
With increasing focus being placed on the long-term impact COVID-19 can have, and the demand for effective treatments also growing concurrently, Marom Bikson (New York City, USA) outlines the potential neuromodulation holds regarding this unmet need.
There is an urgent...
Carthera announced today that its Sonocloud-9 system has been listed as a Breakthrough Device by the Center for Devices and Radiological Health (CDRH) at the US Food and Drug Administration (FDA).
The Sonocloud device uses low-intensity pulsed ultrasound to temporarily...
Next-generation neurointerventional medical device innovator MIVI Neuroscience has announced that Waleed Brinjikji (Mayo Clinic, Rochester, USA) has joined the company as its medical director.
“The MIVI team shares my passion for improving the lives of patients who suffer an acute...
Pooled patient-level data from more than 2,500 asymptomatic, non-octogenarian subjects have shown that carotid artery stenting (CAS) achieved comparable short- and long-term results to carotid endarterectomy (CEA). The findings of this analysis have been published by Jon Matsumura (University...
Pedro Lylyk’s work and achievements in the neurosurgical field have spanned several decades, and range from a momentous intracranial stent placement in 1996, to leading the first in-human study of a novel hydrocephalus treatment in 2022—with more than 10,000...
Deep brain stimulation (DBS) offers a safe therapy option for reducing chorea in patients with Huntington’s disease, but it remains to be seen whether it is superior to any conservative medical treatments in this space. This was the concluding...
New white lesions are not a common occurrence in patients who have undergone transcarotid artery revascularisation (TCAR), a study in which patients were assessed post-procedurally using diffusion-weighted magnetic resonance imaging (DW-MRI), has found.
This was the finding delivered by Raghu...
Craig Weinkauf (Tucson, USA) is leading a trial looking into a connection between Alzheimer's disease and carotid stenosis. "We want to try to quantify the brain: volumes, structural connectivity, functional connectivity and other relevant findings associated with neurodegeneration," he...
Having been launched earlier this year, the Emboguard balloon guide catheter (Cerenovus) was recently used in mechanical thrombectomy procedures for the first time. David Fiorella (Stony Brook University Hospital, New York, USA), who performed these milestone stroke treatments, speaks...
Artio Medical today announced the closing of US$28 million in oversubscribed Series A2 and Series A3 financing, bringing the total amount raised by the company to date to US$74 million.
Funds will be used to support US commercialisation efforts for the recently cleared...
Silk Road Medical today announced a post-market study to evaluate the transcarotid artery revascularisation (TCAR) system in the treatment of standard surgical risk patients with carotid artery disease.
The ROADSTER 3 study will fulfil the US Food and Drug...
A randomised controlled trial (RCT) presented at the International Neuromodulation Society (INS) congress (21–26 May 2022, Barcelona, Spain) has demonstrated significant and clinically relevant reductions in the frequency of medically intractable chronic cluster headache attacks following occipital nerve stimulation...
A new American Heart Association (AHA) scientific statement has highlighted the need for more data about sex differences in the use of and response to mechanical thrombectomy for acute ischaemic stroke, and suggests the need for a clear differentiation...
As per the findings of a multicentre, retrospective study, use indications for the Woven EndoBridge (WEB) intrasaccular flow disruption device (Microvention/Terumo) can be safely extended to include sidewall intracranial aneurysms.
The study’s authors detail in Radiology that no significantly...
Nevro Corporation has announced the results from data presentations at the American Diabetes Association (ADA) 82nd Scientific Sessions (3–7 June 2022, New Orleans, USA) supporting the use of 10kHz spinal cord stimulation (SCS) therapy for patients with chronic pain, including results from the...
This advertorial, sponsored by Medtronic, is intended for readers outside of the USA.
Mechanical thrombectomy may be the standard of care for acute ischaemic strokes caused by a large vessel occlusion (LVO), but even this established and trusted procedure fails...
MindMaze—in partnership with the University of Auckland (Auckland, New Zealand)—has announced a new study that will aim to show the merits of early, immersive and intensive intervention to recover movement for patients who have experienced a stroke.
The Phase 2...
In a randomised, sham-controlled clinical trial, multisession transcranial direct current stimulation (tDCS) paired with concurrent cognitive remediation training has been shown to promote social functioning in individuals with autism spectrum disorder (ASD). These findings, derived from a total of...
Route 92 Medical has announced the publication of initial results from the SUMMIT NZ clinical trial—a single-arm, multicentre, prospective trial evaluating its proprietary Monopoint operating platform.
Results from the first 45 patients demonstrated that the Monopoint platform yielded an...
Neuros Medical has announced the completion of its QUEST pivotal trial’s 90-day primary endpoints, according to a company press release. QUEST outcomes will remain blinded through the 12-month follow-up period, per protocol, the release adds.
QUEST is a 180-subject, randomised,...
Silk Road Medical recently announced that the Centers for Medicare and Medicaid Services (CMS), through collaboration with the Society of Vascular Surgery’s Patient Safety Organization (SVS PSO) and their Vascular Quality Initiative (VQI), has expanded coverage for transcarotid artery...
A myriad of potentially groundbreaking clinical trials were in focus on NeuroNews throughout the month of May. A novel device in pain management, a new frontier in the treatment of basilar artery occlusion stroke, and a significant step forward...
The risk of having a future stroke caused by asymptomatic carotid stenosis is “so low” that most patients with this condition could potentially be treated with the newest medications and may not require surgery. That is according to new...
Axonics has filed a premarket approval (PMA) supplement with the US Food and Drug Administration (FDA) for its fourth-generation rechargeable sacral neuromodulation (SNM) implantable neurostimulator, which is intended to treat bladder and bowel dysfunction.
The fourth-generation stimulation technology reduces how...
Rapid Medical announced today the enrolment of the first patient in the DISTALS study, which the company claims is the first-ever US Food and Drug Administration (FDA) investigational device exemption (IDE) trial to examine the safety and effectiveness of...
Nevro Corporation has announced that data from the SENZA painful diabetic neuropathy (PDN) randomised controlled trial (RCT)—which the company says is the largest RCT to evaluate spinal cord stimulation (SCS) to treat PDN—will be presented at the American Diabetes Association (ADA)...
In December 2021, University Hospital Basel (Basel, Switzerland) enrolled the first patient worldwide in the DISTAL study—a multicentre randomised controlled trial (RCT) evaluating the clinical efficacy of endovascular therapy (EVT) in acute ischaemic stroke patients with an isolated medium...
A novel approach to spinal cord stimulation (SCS), which involves targeting the dorsal horn dendrite—in contrast with more traditional ‘axonal’ SCS techniques—has been labelled as a “true breakthrough” in the field of pain management. That is according to Marc...
As a principal investigator for the pivotal VNS-REHAB study, which supported the first US Food and Drug Administration (FDA) premarket approval of a vagus nerve stimulation (VNS) system for stroke rehabilitation therapy last year, Jesse Dawson (Glasgow, UK) is...
Audience polling during the Acute Stroke Challenges session at this year’s Charing Cross Symposium (CX 2022; 26–28 April, London, UK and virtual) revealed scepticism regarding the benefits of transcarotid artery revascularisation (TCAR) in the treatment of symptomatic carotid artery...
Highlights:
Late-breaking presentations from the European Stroke Organisation Conference (ESOC) progress clinical data on tenecteplase as an alternative intravenous thrombolysis drug to alteplase, and the treatment of basilar artery occlusion stroke with endovascular therapy
Johanna Fifi (New York, USA) and...
Highlights:
Late-breaking presentations from the European Stroke Organisation Conference (ESOC) progress clinical data on tenecteplase as an alternative intravenous thrombolysis drug to alteplase, and the treatment of basilar artery occlusion stroke with endovascular therapy
Johanna Fifi (New York, USA) and...
Neuro42 has announced an exclusive licensing agreement with Johns Hopkins University (Baltimore, USA) for its magnetic resonance (MR)-compatible robot for head, neck and spine applications, with additional non-exclusive licenses for related technologies and patents.
Developed by Dan Stoianovici (Johns...
Osama O Zaidat (Toledo, USA) speaks to NeuroNews about mechanical thrombectomy and the COMPLETE registry dataset—recently published in the Stroke journal —which was designed to evaluate the safety and efficacy of the Penumbra System (Penumbra) in a real-world setting.
The system was shown to be...
The European Society for Vascular Surgery (ESVS) has just released 2023 clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. The guideline document, authored by chairperson Ross Naylor (Leicester Vascular Institute, Leicester, UK), co-chairperson Barbara...
Mohammed Elamir (The Villages, USA) and Amir Hadanny (Be'er Ya'akov, Israel) mark this year’s Stroke Awareness Month by discussing hyperbaric oxygen therapy (HBOT)—an emerging approach that they believe could break new ground in post-stroke recovery.
Every year, more than 800,000...
Adept Medical says it has responded to the call from interventional radiologists for a device to comfortably support and manage a patient in the prone position with the launch of its new Prone Support solution, which will be available...
Vesalio recently announced that it has oversubscribed its Class A financing round. Solas BioVentures led the investment with participation by new and existing investors, including the recent addition of Orlando Health Ventures.
With an expanding customer base established in Europe,...
A new study has identified strains of gut microbiota that are associated with more severe strokes and worse post-stroke recovery, revealing that the gut microbiome could be an important factor in stroke risk and outcomes.
The study, presented at the...
At the time of carotid endarterectomy (CEA), surgeons should consider single antiplatelet therapy (SAPT) rather than dual antiplatelet therapy (DAPT). This is the conclusion of a new meta-analysis—reportedly the largest conducted on the topic to date—published in the European...
Flow Neuroscience, which claims to be the creator of Europe’s first medically approved brain stimulation device to treat depression at home, has received full US Food and Drug Administration (FDA) investigational device exemption (IDE) to launch a US pivotal...
Medtronic today announced the first patient implant in a clinical study of its investigative, closed-loop, implantable neurostimulator. The ‘Evaluation of long-term patient experience with a Medtronic closed-loop SCS system’ study is being conducted in Australia, and its objective...
The risk of being diagnosed with a new cancer in the follow-up of patients with a first-ever stroke is particularly increased in patients younger than 50 years of age, while this risk is similar to that of the general...
MicroTransponder recently announced the first commercial use of its Vivistim paired vagus nerve stimulation (VNS) system for improved upper-limb function post-stroke. The company said in a press release that a surgical team consisting of Charles Liu and Jonathan Russin...
Vesalio has announced the completion of patient enrolment for the CLEAR US Food and Drug Administration (FDA) investigational device exemption (IDE) study utilising the NeVa thrombectomy platform.
In a press release, Vesalio states that this is an important milestone for...
Avail Medsystems has announced that it has entered into a partnership with the Stroke Thrombectomy and Aneurysm Registry (STAR), led by Alejandro Spiotta (Medical University of South Carolina , Charleston, USA), which will attempt to leverage the company’s surgical...
Health Canada recently authorised the Dolphin vagal nerve stimulator to treat symptoms in patients with COVID-19 ‘long-haul’ symptoms (long haulers). Long haulers experience a wide variety of aftereffects from the virus, including shortness of breath, cough, fatigue, joint and muscle pain, headaches, and...
Novel neurovascular technologies, from robotics, to microcatheters, to artificial intelligence (AI), were among the hottest topics featured on NeuroNews in April. A momentous company acquisition worth €500 million and an exclusive interview with Society of NeuroInterventional Surgery (SNIS) president...
Synchron has announced enrolment of the first patient in the US COMMAND clinical trial for patients with severe paralysis at Mount Sinai Hospital in New York, USA. The trial is being conducted under what Synchron claims is the first...
This year’s European Stroke Organisation Conference (ESOC 2022; 4–6 May, Lyon, France) saw results from ATTENTION—a multicentre randomised controlled trial (RCT) evaluating the benefit of endovascular therapy (EVT) in basilar artery occlusion (BAO) stroke patients—presented for the first time....
Against the backdrop of remote learning technologies becoming increasingly important within medical training during the COVID-19 pandemic, a new study has found that—in a remote environment—an artificial intelligence (AI) tutoring system can outperform expert human instructors.
The Neurosurgical Simulation and...
The existing evidence on whether women with carotid stenosis experience inferior treatment outcomes compared to men is mixed—and this uncertainty is reflected in the current clinical guidelines. Seemant Chaturvedi (Baltimore, USA) assesses this evidence and outlines how it may...
Clark Zeebregts (University Medical Centre Groningen, Groningen, The Netherlands) and Kosmas Paraskevas (Central Clinic of Athens, Athens, Greece) answer some key questions about the 2023 European Society for Vascular Surgery (ESVS) clinical practice guidelines on the management of atherosclerotic...
NeuroPace has announced that the first patient was recently treated in the RESPONSE clinical trial, which is evaluating the safety and effectiveness of the RNS system in adolescent patients with drug-resistant focal epilepsy. The procedure took place at Westchester Medical...
Stroke experts have identified how radiomics—an emerging, image-quantifying technology—can be used to extract biomarkers from clinical brain magnetic resonance imaging (MRI) scans in stroke patients and estimate their relative ‘brain age’. The technique demonstrates that using relative brain age,...
A retrospective study involving more than 200 patients has indicated that ‘bridging thrombolysis’ may not be associated with improved functional outcomes in stroke patients who achieve complete recanalisation—as compared to treatment with endovascular therapy (EVT) alone. As such, researchers...
Using a one-millimetre-sized wireless implant to stimulate peripheral nerves from within blood vessels has the potential to treat neuropathic pain that is resistant to traditional medical therapy, according to a team of multi-institutional researchers led by Sunil Sheth (University...
Silk Road Medical recently announced that the US Food and Drug Administration (FDA) approved expanded indications for the company's Enroute stent to include patients at standard risk for adverse events from carotid endarterectomy (CEA). Previously, the stent was approved...
Neurosurgery experts from the University of Cambridge (Cambridge, UK) have led what is claimed to be the largest ever study examining the surgical management of traumatic brain injury, highlighting regional inequalities in both major causes and treatment of such...
Only a small proportion of neuropathic pain patients in England who are potentially eligible for spinal cord stimulation (SCS) have received this intervention over the past decade, as per data from a retrospective cohort study published in Neuromodulation: Technology...
Avicenna.AI has announced a signed distribution agreement with Sectra—an international medical imaging information technology (IT) and cybersecurity company. The agreement will see Avicenna’s AI solutions for neurovascular pathologies offered through the Sectra Amplifier Marketplace, which is a platform for...
ZAP Surgical Systems announced today that Baptist Health South Florida (Miami, USA) will acquire the ZAP-X gyroscopic radiosurgery platform for cranial stereotactic radiosurgery (SRS). The ZAP-X will be used by Miami Neuroscience Institute, in collaboration with Miami Cancer Institute—both...
CereVasc has announced treatment of the first patient in its clinical study of the eShunt system in patients with normal-pressure hydrocephalus (NPH).
Following approval from Argentina's National Administration of Drugs, Foods and Medical Devices (ANMAT) to conduct the second study of...
Engineers from the Massachusetts Institute of Technology (MIT) in Cambridge, USA have developed a telerobotic system to enable surgeons to quickly and remotely treat patients experiencing a stroke or aneurysm. With a modified joystick, surgeons in one hospital may...
GT Medical Technologies announced today that new data on the company's innovative GammaTile therapy for patients with operable brain tumours will be presented via an oral, live presentation at the 2022 American Association of Neurological Surgeons (AANS) annual scientific...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities as chief clinical and regulatory officer, aligning and integrating the company's scientific, medical, clinical research...
Abbott today announced that it has launched an upgraded version of its NeuroSphere myPath digital health app with enhanced functionality intended to help doctors more closely track their patients as they trial the company’s neurostimulation devices to address their...
With an educational event entitled “Innovations in Neuromodulation”, the International Neuromodulation Society (INS) will attempt to bring together industry leaders, innovators and investors to discuss commercialisation from a global perspective at its 15th world congress next month (21–26 May...
Microvention, a wholly owned subsidiary of Terumo Corporation, has announced the successful completion of its first patient enrolment in a multicentre, prospective, observational study called STRAIT.
The European Union (EU) study will evaluate the safety and performance of the company’s new...
Stimwave Technologies has announced the launch of its level-one FREEDOM clinical trial series—which consists of multicentre, prospective, randomised trials that monitor chronic pain patients’ responses to peripheral nerve stimulation (PNS) over time. Upon completion of this series, the study...
Wallaby Medical today announced that it has acquired phenox GmbH—including phenox's femtos GmbH—for a total consideration of approximately €500 million including milestone payments.
The acquisition is one of the largest cross-border transactions in the medical device industry globally in recent years,...
According to new research published in Stroke, about one third of people who had a severe ischaemic stroke—and underwent a mechanical thrombectomy to remove their large-vessel blood clot—resumed work three months after stroke treatment. In addition, women were about...
In March 2022, more than 390,000 people who called 999 with emergencies including suspected stroke waited for an average of over one hour for an ambulance to arrive, as per recent data from the UK National Health Service (NHS).
Ambulance...
NeuraLace Medical has announced the expansion of its global patent portfolio with the issuance of two new patents: US Patent No. 11,305,130 for "Devices, systems, and methods for non-invasive chronic pain therapy" (issued 19 April 2022) and US Patent No. 11,273,317...
Axonics today announced the comprehensive launch of the Axonics F15 across the USA. The newly developed, long-lived, fully recharge-free sacral neuromodulation (SNM) system also received US Food and Drug Administration (FDA) approval in March.
“The Axonics recharge-free system is a welcome advancement...
Bendit Technologies has received US Food and Drug Administration (FDA) 510(k) clearance of its Bendit21 microcatheter for treatment in the neuro, peripheral and coronary vasculature. The clearance was received several months after the successful first use of the Bendit21...
As per early findings from the CHOICE randomised controlled trial (RCT), the use of adjunct, intra-arterial alteplase—among patients with large vessel occlusion (LVO) acute ischaemic stroke and angiographically successful reperfusion following thrombectomy—may lead to a greater likelihood of an...
Corindus, a Siemens Healthineers company, announced today the opening of its new headquarters at 275 Grove Street in Newton, USA. Siemens Healthineers relocated the Corindus headquarters from its previous location in Waltham, USA to accommodate company growth, doubling the...
Barbara Rantner (Munich, Germany) previews the Acute Stroke Challenges programme at CX 2022—a two-hour session featuring several debates and audience polls on carotid disease treatments—and outlines what these discussions hope to achieve.
It is a pleasure for us that the...
Viz.ai has raised a US$100 million funding round at a US$1.2 billion valuation, as per a company press release. The Series D round, which comes as the number of hospitals using the Viz platform surpasses the 1,000 mark, and...
Truvic Medical, a wholly owned subsidiary of Imperative Care, announced in a press release that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Prodigy thrombectomy system, designed for the treatment of peripheral...
Michael Chen—a neurointerventionist and professor of Neurology, Neurosurgery and Radiology at Rush University Medical Center in Chicago, USA—speaks to NeuroNews to provide insight on his career, the ever-evolving world of neurointervention, and also his role in ongoing efforts to...
A new concept for navigating the neurovasculature, performed without the aid of a guidewire, has demonstrated its ability to be manoeuvred through tortuous vessels, braided stents and occlusions, and to deliver and move coils, in preclinical investigations involving a...
Aidoc has received US Food and Drug Administration (FDA) 510(k) clearance for the triage and notification of brain aneurysm—which affects one in 50 people in the USA each year and for which rupture is associated with a 50% chance...
Industry-fronting experts from the worlds of neurointerventional surgery and neuromodulation alike weighed in on a number of key topics throughout the month of March. Transradial access, and its place in endovascular neurosurgery, as well as artificial intelligence (AI) and...
Cefaly Technology has announced the results of a clinical study demonstrating that two-hour treatment with its external trigeminal nerve stimulation (e-TNS) Cefaly device is a safe and effective alternative to pharmaceuticals for the acute treatment of migraine attacks in...
Data from a multicentre clinical study supporting the use of Anaconda Biomed’s Advanced Neurovascular Access (ANA) catheter system for treating ischaemic stroke have been published in the journal Stroke. The company’s mechanical thrombectomy system was shown to achieve a...
Rishi Gupta (Wellstar Neuroscience Institute, Marietta, USA) presents the first analysis of the ASSIST registry, a Stryker-sponsored study that collected real-world data on thrombectomy techniques using Stryker’s Total Stroke portfolio. This core lab adjudicated study enrolled 1,500 subjects across...
The London Alzheimer’s Clinic on Harley Street (London, UK) has become the first clinic in the UK to offer transcranial pulse stimulation (TPS)—a “revolutionary” new neuromodulation treatment for Alzheimer’s dementia—according to an Alzheimer’s Clinics press release.
TPS involves passing short,...
Vitor Pereira and Nicole Cancelliere (St Michael’s Hospital, Toronto, Canada) outline recent breakthroughs in the deployment of robotics for neurointerventional procedures and discuss future use indications for these technologies within stroke treatment.
Although use of robotics in surgery is...
This advertorial is sponsored by Medtronic.
Upon gaining CE-mark approval in September of last year, the Rist radial access system (Medtronic) became the first dedicated technology on the European market providing interventionists with the option of accessing their patients’ neurovasculature...
Biomodex has announced the launch of new clear cartridges for use with all of its EVIAS (Endovascular intracranial aneurysm system) and EVIAS Plus stations for preprocedural 3D simulation. The anatomically accurate clear cartridges are 3D-printed using a transparent material that allows...
Highlights:
Japanese study—first presented at the International Stroke Conference (ISC)—sheds light on mechanical thrombectomy in severe ischaemic stroke patients
The pros and cons of transradial access in neurointerventional surgery discussed by Pascal Jabbour and Kareem El Naamani (Philadelphia, USA)
...
Highlights:
Japanese study—first presented at the International Stroke Conference (ISC)—sheds light on mechanical thrombectomy in severe ischaemic stroke patients
The pros and cons of transradial access in neurointerventional surgery discussed by Pascal Jabbour and Kareem El Naamani (Philadelphia, USA)
...
Researchers at the Wyss Center for Bio and Neuroengineering (Geneva, Switzerland), in collaboration with the University of Tübingen (Tübingen, Germany), have enabled a person with complete paralysis, who cannot speak, to communicate via an implanted brain-computer interface (BCI).
This breakthrough...
Synchron has announced the results from a study in which four people with amyotrophic lateral sclerosis (ALS) received an implant of the company’s Stentrode device—a small, mesh-like material inserted within a patient’s blood vessel that does not require invasive,...
A vascular surgery research team with a growing reputation for its work investigating appropriateness in care in the peripheral vascular bed recently uncovered an association between increased regional market competition among proceduralists and higher odds of revascularisation being carried...
Carthera has announced today the publication of results from an investigator-sponsored pilot clinical trial evaluating safety and efficacy using its Sonocloud technology in patients with mild Alzheimer’s disease. These results are published in the journal Alzheimer’s Research and Therapy.
“The...
Carotid endarterectomy (CEA) does not appear to either reduce or increase the risk of dementia, despite it having been shown to reduce stroke risk. This is according to 20-year results from the ACST-1 trial, published online ahead of print...
Saluda Medical has finalised US$125 million in equity financing, with the proceeds being used in part to operationalise and scale commercialisation of the company’s Evoke spinal cord simulation (SCS) system for chronic pain.
The equity financing was led by existing investor Redmile...
Cerus Endovascular has received US Food and Drug Administration (FDA) 510(k) clearance for its 027 microcatheters, available in two lengths, expanding on a product portfolio that includes the already FDA-cleared 021 microcatheter platform.
The company now expects to submit...
A new survey commissioned by Medtronic and conducted by public opinion research firm The Harris Poll has found that 44% of current chronic back and leg pain sufferers have experienced care delays during the COVID-19 pandemic—despite 87% reporting that...
In the fourth and final instalment of a NeuroNews video series, Jeffrey Saver (University of California, Los Angeles Medical Center, Los Angeles, USA) outlines why a diffusion of responsibility—as compared to more traditional, in-person care approaches—is one of...
Jeffrey Saver (University of California, Los Angeles Medical Center, Los Angeles, USA) details what he feels is the “greatest concern” when it comes to the implementation of robot-assisted systems and remote care protocols within neurointerventional practices—potential harm to...
Jeffrey Saver (University of California, Los Angeles Medical Center, Los Angeles, USA) highlights financial benefits, the phenomenon of ‘training time bias’, and a number of other potential conflicts of interest as important factors that apply to neurointerventionists deploying...
Medtronic recently announced the first patients have been implanted in the TITAN 2 pivotal study—which will evaluate the safety and efficacy of its investigational tibial neuromodulation (TNM) device in people with overactive bladder (OAB). The minimally invasive, implantable technology...
In the first instalment of a four-part video series on NeuroNews, Jeffrey Saver (University of California, Los Angeles Medical Center, Los Angeles, USA) introduces the topic of robotics in neurointerventional surgery and outlines the ethical implications such technologies...
Following the recent introduction of key data supporting the use of high-frequency spinal cord stimulation (SCS) in the treatment of non-surgical refractory back pain (NSRBP), Leonardo Kapural (Carolinas Pain Institute, Winston-Salem, USA) details what these findings mean for a...
In patients with intracranial atherosclerosis, the use of medications and exercise is more beneficial in preventing a second stroke than placing a stent in the blood vessel, according to a new practice advisory issued by the American Academy of Neurology (AAN)....
Stimwave Technologies has provided an update on recent reimbursement-related progress pertaining to its chronic pain therapies—which include implantable spinal cord stimulation (SCS) and peripheral nerve stimulation (PNS) technologies.
In February 2022, the American Medical Association’s current procedure terminology (CPT) editorial...
AiM Medical Robotics recently announced that it has raised US$3.4 million in seed funding, led by IQ Capital and Surrey Capital Partners. Other investors include The Lambda Fund, Triple Ring Technologies, and Worcester Polytechnic Institute, among additional inside investors.
As of the close...
Zeta Surgical has announced the close of US$5.2 million in seed financing—and will use these funds to finalise development of its neurosurgical navigation platform, Zeta, and its non-invasive focused ultrasound system, ZetaFUS.
The completion of initial clinical studies, obtaining US Food...
Saluda Medical has announced that it has received full approval from the US Food and Drug Administration (FDA) for the Evoke spinal cord stimulation (SCS) system, which is indicated for the treatment of chronic intractable pain of the trunk and/or...
Alejandro Spiotta (Medical University of South Carolina, Charleston, USA) outlines what he believes could be the next frontier in cerebral aneurysm care—artificial intelligence (AI)—and the specific benefits it holds in this space.
Endovascular treatments for brain aneurysms and other cerebrovascular...
Lower frequencies of dorsal root ganglion stimulation (DRG-S) may be more effective in the treatment of neuropathic pain—as per a late-breaking presentation from Guilherme Santos Piedade (University of Düsseldorf, Düsseldorf, Germany) at this year’s North American Neuromodulation Society (NANS)...
As the adoption of radial access across the world of neurointerventional surgery begins to pick up pace, Pascal Jabbour and Kareem El Naamani (Thomas Jefferson University, Philadelphia, USA) take a look at both sides of the coin in this...
A new strategy using telehealth to monitor blood pressure at home for several months immediately after a stroke had a positive impact on patient engagement and blood pressure control among people who live in historically under-resourced communities, according to preliminary research presented at...
In patients with treatment-resistant depression (TRD), multiple sessions of intermittent theta-burst stimulation (iTBS) do not accelerate responses to the therapy, nor do they improve depressive symptoms. This is the key finding of the CARTBIND (Canadian rTMS treatment and biomarker...
PathMaker Neurosystems has announced initiation of its US multicentre clinical trial to evaluate MyoRegulator for the non-invasive treatment of post-stroke upper-limb spasticity. The trial is funded by a US$4.9 million Cooperative Research to Enable and Advance Translational Enterprises for Devices...
Following the introduction of its BrainPath navigation probe adapter at the International Stroke Conference (ISC 2022; 9–11 February, New Orleans, USA), medical device innovator NICO Corporation claims to have become the first and only provider to offer an adapter for electromagnetic navigation...
Axonics has announced that the US Food and Drug Administration (FDA) has approved its newly developed, long-lived, recharge-free sacral neuromodulation (SNM) implantable neurostimulator, the Axonics F15 system, for adult patients with bladder and bowel dysfunction.
In a press release, Axonics...
Rapid Medical has announced the promotion of Eitan Havis to international president of the company. Havis will oversee the commercial expansion of the company’s advanced neurovascular technologies to treat haemorrhagic and ischaemic stroke patients in Europe, Asia, and other geographies.
According to...
Medtronic has announced that it has entered into a contract with Vizient to add Touch Surgery Enterprise, an AI-powered surgical video management and analytics platform for the operating room (OR), to Vizient's offerings.
In a press release, Medtronic said that...
Valencia Technologies Corporation has announced that the US Food and Drug Administration (FDA) has granted premarket approval of its eCoin leadless tibial neurostimulator for the treatment of urinary urge incontinence (UUI)—which affects more than 60% of patients who suffer...
On technological and commercial fronts alike, the neuromodulation space is one of the more rapidly evolving subsectors within the medical industry—and has undergone a remarkable period of growth in the past couple of decades. With this progress showing little...
At the International Neuromodulation Society (INS) 15th world congress (21–26 May 2022; Barcelona, Spain), researchers from over 40 countries will present plenary lectures and more than 470 abstracts about neuromodulation therapy and research—as per an INS press release.
In the...
A spinal cord stimulation (SCS) system capable of simultaneously delivering multiple treatment modalities has produced a sustained improvement in outcomes in a randomised controlled trial (RCT) involving chronic pain patients. Two-year findings from this study—the COMBO (Combining mechanisms for...
InspireMD has announced that its CGuard embolic prevention stent system (EPS) will be included as a device option for stenting in CREST-2 (Carotid revascularisation endarterectomy or stenting trial). Following the recent approval of the CREST-2 investigational device exemption (IDE)...
Highlights from the International Stroke Conference (ISC 2022; 9–11 February, New Orleans, USA)—predominantly relating to mechanical thrombectomy studies—grabbed our readers’ attention throughout February. New introductions to the stroke care field, including the Emboguard balloon guide catheter (Cerenovus) in acute...
NeuroOne Medical Technologies has announced that it successfully completed initial, bench-top, long-term stimulation testing on a novel design of its thin-film electrode technology. The tests measured the electrodes' ability to deliver the number of electrical stimulation pulses required to...
LivaNova has announced the first patient has been implanted as part of an investigational device exemption (IDE) clinical study, dubbed OSPREY (Treating obstructive sleep apnea using targeted hypoglossal neurostimulation). The randomised controlled trial (RCT) seeks to demonstrate the safety and effectiveness...
Accuray has announced that the National Institute for Health and Care Excellence (NICE) has endorsed the use of its radiosurgery technology for trigeminal neuralgia—strengthening evidence for the company to market its CyberKnife treatment delivery system for this indication in...
Microvention, a subsidiary of Terumo, has announced the first US clinical case using its next-generation flow diverter, the FRED X device, at Thomas Jefferson University Hospital (Philadelphia, USA). The FRED X flow diverter features Microvention X technology—a proprietary nano-polymer surface modification...
Johns Hopkins Medicine researchers have developed and tested a new imaging approach they say will accelerate imaging-based research in the lab by allowing investigators to capture images of blood vessels at different spatial scales.
Tested in mouse tissues, the method,...
Charing Cross (CX) chair Roger Greenhalgh welcomes the vascular community to this year's symposium, due to be held 26–28 April in London, UK, and virtually. To read the preliminary programme and register visit cxsymposium.com.
Viz.ai has received US Food and Drug Administration (FDA) 510(k) clearance for Viz ANEURYSM—a new algorithm that uses artificial intelligence (AI) to detect suspected cerebral aneurysms. According to a company press release, this is intended to help hospital systems...
In spite of the clinical successes observed to date, a lack of high-level safety and efficacy evidence, among other current challenges and limitations, must be overcome in order to realise the potential held by robotics in neurointerventional surgery. This...
Aleva Neurotherapeutics has received a letter of approval from the US Food and Drug Administration (FDA) for an investigational device exemption (IDE) study assessing the company’s directSTIM directional deep brain stimulation (DBS) system—which is intended for use in major...
Medtronic has announced receipt of approval from the US Food and Drug Administration (FDA) for its new, recharge-free InterStim system—with the device being made available immediately for the treatment of overactive bladder, chronic faecal incontinence, and non-obstructive urinary retention.
"Countless...
Serious disability is six times more likely to be the result three months after stroke among children whose large-vessel stroke was not treated with a mechanical thrombectomy procedure, according to preliminary late-breaking research presented the International Stroke Conference (ISC...
Neuromod Devices has welcomed the findings of an independent study performed at the German Hearing Center at Hannover Medical School (Hannover, Germany), which found that 85% of tinnitus patients experienced a reduction in their tinnitus symptoms—based on the Tinnitus...
When comparing outcomes for acute ischaemic stroke patients treated at various levels of stroke centres, patients who received care at comprehensive stroke centres (CSC) or thrombectomy-capable stroke centres (TSC) were more likely to receive rapid treatment with clot-busting medication and/or mechanical...
Despite its small sample size, a study in which a patient received closed-loop, deep brain stimulation (DBS) therapy has demonstrated positive clinical outcomes and indicates the potential held by individualised, biomarker-driven neuromodulation for treatment-resistant depression. This is according to...
Myomo has announced that new research measuring the benefits of its Myopro myoelectric orthosis found “statistically significant improvements” in a number of motor function measurements. The research study, titled “Myoelectric arm orthosis in motor learning-based therapy for chronic deficits...
A relatively new introduction to the field of endovascular therapy (EVT) for acute ischaemic stroke, the Skyflow stent retriever (Skyflow), has demonstrated non-inferiority with the Solitaire FR revascularisation device (Medtronic) in a randomised controlled trial (RCT)—the results of which...
A new peer-reviewed study published in Pain Medicine, examining the use of Nerivio’s (Theranica) guided imagery, education and relaxation (GIER) in-app optional behavioural intervention feature, has demonstrated a positive impact for the acute treatment of migraine.
The GIER feature is...
In a phase 2a clinical trial in China, the clot-busting medication tenecteplase has been shown to be effective in restoring blood flow to the brain without symptomatic brain bleeding—as per a late-breaking presentation at the recent International Stroke Conference...
Rapid Medical has announced US Food and Drug Administration (FDA) Breakthrough Device designation for its Comaneci embolisation assist technology, which is intended to facilitate the treatment of cerebral vasospasm—a major complication and cause of morbidity from subarachnoid haemorrhage—following a haemorrhagic stroke.
“We...
Imperative Care has announced the launch of its Zoom POD aspiration tubing—the company’s latest innovation in elevating stroke care.
This is the newest addition to Imperative’s Zoom stroke solution, which is an ischaemic stroke product portfolio also including the...
Nevro Corporation has announced online publication of 12-month data from the SENZA-NSRBP randomised controlled trial (RCT) in Journal of Neurosurgery: Spine. These data show that high-frequency 10kHz spinal cord stimulation (SCS) therapy results in profound improvements in non-surgical refractory back...
Mechanical removal of blood clots reduced post-stroke disability in nearly half of “all-comer” real-world stroke patients in a global study, according to preliminary late-breaking research presented at this year’s International Stroke Conference (ISC 2022; 9–11 February, New Orleans, USA).
“This...
NovaSignal Corporation has announced the results of a multicentre, prospective, single-arm study indicating that the company’s autonomous NovaGuide intelligent ultrasound is three times more likely to detect right-to-left shunt (RLS)—a recognised risk factor for stroke—than standard of care transthoracic...
Data from several new studies presented at the recent International Stroke Conference (ISC 2022; 9–11 February, New Orleans, USA) further validate the “best-in-class” sensitivity and specificity of Viz.ai’s intelligent care coordination platform for stroke in real-world settings across multiple commercial modules—according to...
NeuroOne Medical Technologies has announced that it successfully completed initial preclinical, long-term testing of recording capabilities on its thin-film platform electrode technology.
In test procedures, the electrodes' ability to record electrical activity over five years was measured using an...
An analysis of the worldwide experience of transcarotid artery revascularisation (TCAR) has produced key objective proficiency metrics and an analytic framework to assess adequate training for the procedure. “Training on cadavers or synthetic models achieved clinical outcomes, technical outcomes...
Cerenovus—a neurovascular firm that forms part of Johnson & Johnson Medical Devices Companies—has announced the launch of Emboguard, its next-generation balloon guide catheter to be used in endovascular procedures, including those for patients with acute ischaemic stroke.
Emboguard is designed...
A new study from Japan has become the first randomised controlled trial (RCT) to demonstrate the effectiveness of endovascular mechanical thrombectomy procedures in patients who have severe strokes involving clots in one or more large brain arteries. This preliminary,...
An exclusive interview with the North American Neuromodulation Society (NANS) president piqued the interest of NeuroNews’ readers through the first month of the year, as did fresh research into the possibilities enabled by new flow diverter devices, 3D-printed aneurysm...
Perfuze announced today that it has closed a €22.5 million Series A investment round—the proceeds from which will be used to drive the next stage of a US clinical study and regulatory clearance of its Millipede system. The funds...
A new research letter in Stroke outlines use of the novel National Institutes of Health (NIH) ‘All of Us’ research programme dataset of diverse participant data to identify factors that increase the risk of carotid artery stenosis and those...
CereVasc has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) application to initiate a pilot trial of the eShunt system in patients with normal-pressure hydrocephalus (NPH).
"This is a very important stage...
Route 92 Medical has announced the first patient enrolment in its SUMMIT MAX clinical trial—a randomised, controlled, multicentre study to evaluate the performance of its next-generation Monopoint reperfusion system versus currently available aspiration catheter technology. The first patient was...
A retrospective analysis of a prospectively maintained database has found that robot-assisted carotid artery stenting (CAS) is both safe and effective. Writing in Neurosurgical Focus, the analysis’ authors also state that the learning curve for robotic procedures was overcome...
The findings of a randomised controlled trial (RCT) have indicated that accelerated intermittent theta-burst stimulation (iTBS), administered to the left dorsolateral prefrontal cortex (DLPFC), is an effective and well-tolerated therapeutic approach in patients with Alzheimer’s disease. The results of this trial...
Vesalio has announced the addition of Earl Slee as a board observer and senior advisor to the company, effective immediately. According to a company press release, Slee brings more than 35 years of medical device innovation and business development...
Vena Medical has announced the first Health Canada medical device licence for its new product, the Vena balloon distal access catheter, which combines the balloon guide catheters and distal access catheters that are currently used in thrombectomy to remove...
Michael Chen (Rush University Medical Center, Chicago, USA) discusses the growing awareness around the need for timely interventions in stroke care in the USA, and outlines efforts being undertaken by the Get Ahead of Stroke campaign to address this...
Abbott announced today that UnitedHealthcare (UHC)—the largest private health insurance company in the USA—has updated its ‘Implanted electrical stimulator for spinal cord’ medical policy to expand patient access to Abbott’s dorsal root ganglion (DRG) neurostimulation devices for people suffering...
Center for Pain and Stress Research today announced that its Dolphin vagus nerve stimulation (VNS) device has received breakthrough approval from Health Canada—making it Canada’s first VNS therapy specifically intended for treating COVID-19.
As per the Health Canada authorisation letter,...
In an effort to improve treatment for obsessive-compulsive disorder (OCD), a team of researchers has—for the first time, according to a Brown University (Providence, USA) announcement—recorded electrical signals in the human brain associated with fluctuations in OCD symptoms over...
Galvani Bioelectronics has announced that the first patient with rheumatoid arthritis (RA) has been treated through stimulation of the splenic nerve using its novel bioelectronics platform. This investigational treatment is the first in the clinic of a new class...
RapidAI has announced Karim Karti, former president and CEO of GE Healthcare Imaging, as the company’s chief executive officer (CEO).
“Karim’s tremendous global experience in healthcare technology and digital health makes him an ideal fit for RapidAI. He has a...
Rebus Biosystems, an Illumina Ventures-backed life science technology firm, has announced the publication of a study in Science highlighting its Rebus Esper platform’s ability to spatially resolve vascular cell diversity in the adult human brain. This effort was led by researchers...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As per an FDA press release, the finalised version of the first of these two guidance...
According to a company press release, a recent publication in the European Journal of Radiology concludes that Texray radiation protection equipment reduces radiation dose by up to 97% during clinical interventions. This was the main concluding finding of the...
This year’s North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA) saw 18-month data from SENZA-PDN—a randomised controlled trial (RCT) evaluating the safety and efficacy of high-frequency spinal cord stimulation (SCS) in painful diabetic neuropathy (PDN) patients—disclosed...
Medtronic has received US Food and Drug Administration (FDA) approval of its Intellis rechargeable neurostimulator and Vanta recharge-free neurostimulator for the treatment of chronic pain associated with diabetic peripheral neuropathy (DPN).
Medtronic estimates that up to 800,000 US patients suffer...
A systematic review and meta-analysis of 71 studies suggests that, at present, carotid endarterectomy (CEA) is safer than carotid artery stenting (CAS) when performed within two or seven days of the index event. “The findings of this analysis will...
Brainomix has announced the launch of e-Stroke 11, the latest version of the company's AI-powered stroke imaging platform. According to a Brainomix press release, this will enhance stroke network communications by allowing physicians to quickly and securely access, review...
Salim Hayek is chief of the Division of Pain Medicine at University Hospitals of Cleveland and a professor in Case Western Reserve University’s Department of Anesthesiology in Cleveland, USA. Having received his medical degree from the American University of...
Engineers and researchers in the Institute of Bioelectronic Medicine at the Feinstein Institutes for Medical Research have developed a novel, fully implantable, wireless bidirectional vagus nerve stimulation (VNS) and sensing device for research in mice. Details about the device and its capabilities, published...
The National Institutes of Health (NIH) has launched the first phase of the Neuromod Prize—a US$9.8 million competition to accelerate the development of neuromodulation therapies. The competition seeks scientists, engineers and clinicians to submit novel concepts and clinical development plans...
Bendit Technologies has announced the successful first use in the USA of its Bendit21 neurocatheter in a life-saving treatment. The case was completed at the University of Texas Southwestern (UTSW) Medical Center and involved a 57-year-old female patient who had been...
Fresh data from the CERUS study—the first multicentre clinical trial evaluating safety and effectiveness outcomes with the Contour neurovascular system (Cerus Endovascular)—have indicated “encouraging results” in the treatment of unruptured intracranial bifurcation aneurysms.
Reporting these results in the journal...
Theranica has announced a new peer-reviewed study analysing the efficacy, safety and sustainability of remote electrical neuromodulation (REN) as a standalone, and medication-adjunct, treatment of migraine. The real-world evidence study, which is published in Frontiers in Pain Research, concludes that...
Nevro Corporation has announced the results from data presentations at the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA) supporting the use of 10kHz spinal cord stimulation (SCS) therapy for patients with chronic pain. These...
Medtronic has announced three-month results from an on-label, prospective, multicentre study showing meaningful pain relief using DTM SCS endurance therapy—a modified, lower-energy variation of the company's Differential Target Multiplexed (DTM) spinal cord stimulation (SCS) therapy for chronic overall, back or...
COVID-19 may have held a defining role in the “rebirth” of telehealth and remote care models in the USA, but their utility in improving patient access to neuromodulation therapies looks set to extend far beyond the global pandemic. This...
Stanford accelerated intelligent neuromodulation therapy (SAINT)—a novel, high-dose intermittent theta-burst stimulation (iTBS)—was found to be safe and more effective than sham stimulation in a recent randomised controlled trial (RCT). And, according to Nolan Williams, assistant professor within the Department...
The American Academy of Neurology (AAN) has issued a position statement for neurologists on how to navigate consent issues for people who have experienced acute ischaemic stroke. The statement is published in an online issue of Neurology, the medical journal of the...
SynerFuse has announced the first implantation in the company's proof-of-concept study evaluating the safety and tolerability of simultaneously implanting spinal fusion hardware and a dorsal root ganglion (DRG) neurostimulator in patients suffering from chronic lower back pain (CLBP).
The first...
Evasc Neurovascular has announced enrolment of the first patient in its eClips safety, feasibility and efficacy study (EESIS-FR) in France. The study will be conducted with the objective of evaluating the technical feasibility, safety and efficacy of the company’s...
ChenMed has announced results from a three-year study of nearly 60,000 patients, showing 22% lower incidence of primary stroke among those having benefitted from a year or more of ChenMed care. The study compared outcomes for more than 40,500 tenured...
Wavegate Corporation has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for its StimuLux optical reflectometry system for closed-loop adaptive modulation of spinal cord stimulation (SCS).
"We are pleased the FDA has granted Breakthrough Device...
Medtronic has announced its schedule for presentations at the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA). According to a press release, Medtronic therapies and technologies will be represented as part of 23 podium and poster...
Endovascular neurosurgery rehearsals using 3D-printed, patient-specific aneurysm models have demonstrated the ability to optimise embolisation strategy, as per the findings of a study published in Journal of Neuroradiology. This optimisation resulted in shorter procedure duration and cumulative fluoroscopy time,...
In the lead up to the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA), the topic of neuromodulation featured prominently among NeuroNews’ most read stories across December. Coverage of other major conferences in the wider neurology...
Mainstay Medical has announced the publication of two-year patient outcomes data from its pivotal ReActiv8-B clinical trial. The data, which are published in Neuromodulation: Technology at the Neural Interface, confirm the efficacy and safety of the company’s implantable ReActiv8...
Patients who have postoperative neurological deficit following lumbar spine surgery are 22 times more likely to have exhibited intraoperative somatosensory-evoked potential (SSEP) changes. This is according to a new meta-analysis published recently in the journal Spine by Robert Chang...
Saluda Medical today announced long-term results from its double-blind, Level 1 EVOKE trial, which have been published in JAMA Neurology and demonstrate superior, sustained pain relief with the company’s Evoke closed-loop spinal cord stimulation (SCS) technology as compared to open-loop...
Boston Scientific has announced key data that will be featured at the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA). More than 20 abstracts have been accepted, including three late-breaking presentations, according to a press...
Highlights:
Insight from the "largest prospective study to date" of the p64 flow modulation device (phenox)
The latest clinical data on endovascular therapy for stroke, including results from the SWIFT-DIRECT trial and the AURORA meta-analysis' findings
Alejandro Frangi (Leeds,...
Highlights:
Insight from the "largest prospective study to date" of the p64 flow modulation device (phenox)
The latest clinical data on endovascular therapy for stroke, including results from the SWIFT-DIRECT trial and the AURORA meta-analysis' findings
Alejandro Frangi (Leeds,...
iMediSync recently showcased its comprehensive electroencephalogram (EEG) solution, designed to perform brain screening and predictive analysis of potential mental conditions in just 10 minutes, at the Consumer Electronics Show (CES; 5–8 January 2022, Las Vegas, USA).
The company launched its first...
This advertorial is sponsored by Perflow.
The StreamTM family of products (Perflow) is a range of dynamic neurothrombectomy net devices designed to give physicians greater control and visibility when treating ischaemic stroke. The first clinical use of the Stream17—a lower-profile...
Wallaby Medical has partnered with Japan Lifeline (JLL) to bring its Pharmaceuticals and Medical Devices Agency (PMDA)-approved Avenir coil system to the Japanese market. Avenir is used in the intravascular embolisation of intracranial aneurysms and other neurovascular malformations, such...
Abbott has announced that the US Food and Drug Administration (FDA) has approved new, expanded magnetic resonance imaging (MRI) compatibility for its Proclaim XR spinal cord stimulation (SCS) system with Octrode leads. This new labelling lifts MRI restrictions for lead tip location...
A cost-effectiveness model based on peer-reviewed sources suggests that, although five-year costs for transcarotid artery revascularisation (TCAR; Silk Road Medical) were higher than those for carotid endarterectomy (CEA), TCAR afforded greater quality-adjusted life years (QALY).
The study is published in the...
Vesalio recently announced the start of enrolment in its NATURE study using enVast, the company’s first thrombectomy system for patients presenting with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention.
NATURE is a randomised, multicentre clinical trial comparing...
A study intended to gain insight into prevailing ethical issues surrounding the use of deep brain stimulation (DBS) in adolescents with obsessive-compulsive disorder (OCD) has found that concerns relating to the conditions of paediatric patient decision-making, a lack of...
The worldwide incidence and mortality rates for stroke decreased slightly from 1990 to 2019, but the overall numbers remain high—especially in high- and middle-income countries—according to a study published in an online issue of Neurology, the medical journal of the American...
Maricela Schnur (St Luke’s Hospital, Duluth, USA) highlights a handful of socioeconomic disparities, racial differences, and psychological and psychiatric factors, that currently present difficulties in patient access to spinal cord stimulation (SCS).
Barriers and gaps in the delivery of healthcare...
Accuray has received approval from the Japanese Ministry of Health, Labor and Welfare (Shonin) to market the CyberKnife robotic radiosurgery system for the treatment of trigeminal neuralgia (TN) in Japan.
The system is currently used to treat TN in the USA,...
In silico studies involving virtual participants hold great potential in the neurovascular field—and the broader medical industry—with their future role in the generation of scientific evidence likely to be alongside more traditional clinical trials. And, by circumventing some of...
Doctors at St David's Medical Center in Austin, USA recently implanted a new neurostimulator technology in patients to help treat advanced heart failure—and are among the first physicians in the USA to do so, according to a press release.
The...
ClaroNav Kolahi—a subsidiary of ClaroNav—has received regulatory clearance from the National Medical Products Administration (NMPA) to market and sell its Navient cranial model in China. Previously, the company had received NMPA approval for its Navient ENT model.
Navient, an image-guided...
Artificial intelligence (AI) and the role it can play in improving acute ischaemic stroke care protocols took centre stage at the Barts Research and Advanced Interventional Neuroradiology conference (BRAIN 2021; 13–16 December, London, UK). Ameer Hassan, head of the...
BrainQ has announced that the first patient has been enrolled at MedStar National Rehabilitation Hospital (Washington, USA) in the pivotal, randomised, double-blind, multicentre EMAGINE trial, which is evaluating its frequency-tuned electromagnetic field treatment in facilitating recovery in individuals with...
The latest technologies and research breakthroughs in neuromodulation feature prominently among our top stories for November, while two momentous announcements from the neurosurgery field—courtesy of Rapid Medical and Robocath—also garnered plenty of attention throughout the month.
1. NeuroPace gains IDE...
Johns Hopkins Medicine researchers say they have new evidence from mouse experiments that manipulating certain nerve cells, or the genes that control them, with neuromodulation might trigger the formation of new heart muscle cells and restore heart function after...
CereVasc has announced the publication of a case report detailing the first treatment in a study of its eShunt system—an investigational device intended to treat communicating hydrocephalus (CH).
According to a press release from the company, the eShunt device...
Passive recharge burst spinal cord stimulation (B-SCS) can alleviate pain intensity and psychological distress, and improve physical function and health-related quality of life out to two years, according to the latest data from the TRIUMPH study.
This prospective, international, multicentre,...
MicroTransponder today announced that its Vivistim paired vagus nerve stimulation (VNS) system has won the Neurotech Reports Gold Electrode Award for best new product. This announcement comes as MicroTransponder prepares for the first commercial implantation of its Vivistim system and as...
FluxWear has announced that it will be demonstrating its first product at the Consumer Electronics Show (CES) early next year (5–8 January 2022, Las Vegas, USA). The product, SHIFT, is a wearable neuromodulation device that allows those suffering with...
Evidence favouring endovascular therapy (EVT) in anterior circulation stroke patients presenting with reversible cerebral ischaemia in the later time window (beyond six hours) from time last known well has been strengthened by a recent systematic review and individual patient...
Researchers at Toronto Western Hospital in Canada have proposed a protocol that may precede in-person assessments prior to patients receiving neuromodulation therapies. Their protocol incorporates a short mental health checklist and pre-defined cut-offs on validated questionnaires to assess whether...
As part of a recently announced clinical trial, brain signal recording with the Wyss Center for Bio and Neuroengineering’s novel brain-monitoring technology, its subscalp Epios sensing electrodes, is being carried out for the first time in patients at the University...
Nevro has announced three clinical data publications on treating painful diabetic neuropathy (PDN) with 10kHz spinal cord stimulation (SCS) therapy—including 12-month data from the SENZA-PDN randomised controlled trial (RCT) published online in Diabetes Care. In addition, the company announced publication...
The largest multi-institutional international study to date on brain complications associated with COVID-19 has found that approximately one in 100 patients hospitalised following SARS-CoV-2 infection will likely develop complications of the central nervous system. These can include stroke, haemorrhage...
Canon Medical Systems is introducing an all-new size for its high-definition flat panel detector for vascular and interventional radiology procedures, a press release from the company reports. Canon claims that the 12”×16” high-definition detector provides more than twice the...
A new study has found that brain scans taken during the lifetimes of athletes in contact sports, compared to changes in their brains at autopsy, showed that white matter hyperintensities—markers of injury to the brain’s white matter—were associated with...
The Diversion-p64 study—the largest prospective study using the p64 flow modulation device (phenox) to date—has demonstrated that the device has a high level of efficacy and carries a low rate of mortality and permanent morbidity. This is the conclusion...
A research team in South Korea has developed and successfully implanted a microrobot device into the brain tissue of mice through the intranasal pathway, potentially opening up new possibilities in the treatment of intractable neurological diseases in the future.
Hongsoo Choi...
Masimo has announced the findings of a prospective study published in Frontiers in Neurology in which Na Xu and colleagues at Capital Medical University in Beijing, China investigated whether general anaesthesia guided by the company’s SedLine brain function monitoring parameters, and...
Antibiotic impregnated envelopes appear to be a safe and effective modality for decreasing surgical site infection risks in spinal cord stimulation (SCS) implantation, according to study data published in Journal of Pain Research.
The report, which is authored by...
Robocath today announced its successful first robotic carotid stenting at Rennes University Hospital. This breakthrough in the neurovascular field was performed on 16 November 2021, using Robocath’s R-One robot, operated by François Eugène (Rennes University Hospital, Rennes, France) and...
This advertorial is sponsored by Nicolab.
In 2021, the Royal Infirmary of Edinburgh and networking hospitals are implementing StrokeViewer (Nicolab)—which is an artificial intelligence (AI) solution that helps physicians with the triage and diagnosis of stroke patients. Jonathan Downer (University...
Accuray has announced the publication of more than two decades of globally-driven clinical data supporting the use of its CyberKnife robotic radiotherapy platform for the delivery of stereotactic radiosurgery (SRS) treatments for neurological diseases. The most recent CyberKnife data, an analysis...
New data on stroke rehabilitation and chronic migraine therapy caught the eye of NeuroNews' readers in October, as did notable discussions from industry-leading events including CX Aortic Vienna 2021 (5–7 October, broadcast) and the Vascular Annual Meeting (VAM 2021;...
At this year’s Society of Vascular and Interventional Neurology annual meeting (SVIN 2021; 17–20 November, Phoenix, USA and virtual), Viz.ai introduced Viz ANX, a new technology that it claims is the first and only artificial intelligence (AI)-powered suspected cerebral...
A recent study has pointed to an increased risk of stroke in both asymptomatic and symptomatic female patients, as well as readmission in asymptomatic female patients following carotid endarterectomy (CEA) or carotid artery stenting (CAS). These findings were published...
BlueWind Medical has announced the successful completion of patient enrolment in the OASIS pivotal clinical study that will assess the company’s innovative Renova iStim implantable neuromodulation device for the treatment of urinary urgency incontinence, and form the basis on...
A study conducted by neurology researchers at University of Texas Southwestern (UTSW) Medical Center has found lower-quality functional outcomes for Hispanic ischaemic stroke patients who receive endovascular thrombectomies than for comparable white patients. Outcomes were similar between white and...
This advertorial is sponsored by Perflow.
The Cascade Net (Perflow)—a non-occlusive remodelling net used in the treatment of cerebral aneurysms—is the first and only device capable of providing continuous blood flow during these repair procedures, and is designed to allow...
NeuroPace has announced that it has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to initiate the NAUTILUS study—which will assess the company’s RNS system in patients with drug-resistant idiopathic generalised epilepsy (IGE).
IGE is...
New research published in Translational Psychiatry presents evidence that brief intraoperative exposure to therapeutic deep brain stimulation (DBS) at the time of implantation surgery induces rapid and consistent electrophysiological brain state changes within minutes—shedding light on the mechanism of action...
Medtronic has announced its ambition to achieve net zero carbon emissions by fiscal year 2045 across its operations and value chain to accelerate efforts to combat climate change. The announcement comes amidst the 2021 United Nations Climate Change Conference...
Insightec has announced that the US Food and Drug Administration (FDA) has approved the Exablate Neuro device for treating advanced Parkinson's disease patients suffering from mobility, rigidity or dyskinesia symptoms.
The Exablate Neuro uses focused ultrasound waves to precisely target and...
Matthias Volz (University of Kassel, Kassel, Germany), Simon Ladwig and Katja Werheid (both Humboldt-Universität zu Berlin, Berlin, Germany) discuss the burden of post-stroke depression (PSD), and how this complication impacts the re-integration of stroke survivors back into their social...
Nevro Corporation has provided an update on its position following a Delaware jury’s decision to award Boston Scientific US$20 million over patent infringements. The jury found that Nevro infringed two Boston Scientific patents (7,891,085 and 8,019,439) directed to ways of...
Rapid Medical today announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for what it claims is the first ever trial to expand interventional stroke treatment to distal regions of the brain.
The DISTALS study is a...
Rune Labs, a company using aggregated brain data to empower the development and delivery of precision medicines for neurological and psychiatric diseases, and global healthcare firm Medtronic have announced a new project designed to better understand the effects of neurostimulation...
A new study presented at the 13th World Stroke Congress (WSC 2021; 28–29 October, virtual) found that the implementation of Brainomix's e-Stroke software resulted in faster treatment times, enabling more stroke patients to achieve functional independence when compared with a...
Magnus Medical has announced that the US Food and Drug Administration (FDA) has granted the company Breakthrough Device designation for its individualised, rapid-acting, non-invasive neurostimulation technology designed to treat major depressive disorder in people who have not improved sufficiently...
NeoSpine is now offering spinal cord stimulation (SCS) procedures for the treatment of painful diabetic neuropathy (PDN) via its HFX system—a newly approved nondrug treatment for PDN which, according to NeoSpine, is the only SCS system approved by the US...
Increasing levels of experience in performing mechanical thrombectomies have been associated with shorter procedural duration and better reperfusion rates in a multicentre study—with the study’s researchers noting that a “theoretical ceiling effect” was observed after about 100 procedures.
Writing in...
On World Stroke Day—29 October 2021—physicians from the Get Ahead of Stroke campaign are urging lawmakers to update triage and transport protocols. They are also asserting that, if the nation’s stroke system of care looked more like its trauma...
On the eve of World Stroke Day (29 October), the World Stroke Organization (WSO) has called for urgent improvements to the treatment of stroke and increased awareness of symptoms. This comes after a survey it conducted in partnership with...
Phenox has added to its extensive technology portfolio with the introduction of the pRESET 6-50 mechanical thrombectomy device for the treatment of acute large vessel occlusive (LVO) stroke.
This latest addition makes the pRESET 6-50 the longest stent retriever in...
Biomodex, a company specialising in biorealistic haptic simulators for physician training and rehearsals, has announced the launch of Thrombotech—a new, hydrogel-based synthetic clot product that can be used for neurovascular training with the company’s EVIAS Plus training solution for...
Evasc Neurovascular has announced the third generation of the eClips device. The device is innovated for more effective treatment of intracranial bifurcation aneurysms, facilitating treatment for a wider range of aneurysms with shorter delivery times, according to an Evasc...
SPR Therapeutics has obtained clearance from the US Food and Drug Administration (FDA) of a broader indication for the usage of its SPRINT peripheral nerve stimulation (PNS) system. Prior clearance limited use of the SPRINT PNS system to the...
Theranica has announced the publication of a new, peer-reviewed article in Pain Reports detailing a study assessing its novel, drug-free treatment option for chronic migraine. The prospective, multicentre, open-label trial evaluated the efficacy and safety of remote electrical neuromodulation...
North Carolina has updated its emergency stroke care protocol—a move applauded by the Get Ahead of Stroke campaign. The protocol, which went into effect on 15 October 2021, will change the way first responders triage and transport severe stroke...
Neuvotion has announced that it has received more than US$1 million in seed funding. The Long Island Angel Network, an organised group of individual, Long Island-based angel investors, and the Good Shepherd Rehabilitation Network, a nationally recognised rehabilitation leader,...
MindMaze Healthcare has announced the first UK partnership for its novel MindPod platform to target cognitive and motor restoration in stroke patients. This new partnership involves the Royal Buckinghamshire Hospital (RBH), which is based in Aylesbury, UK.
“MindPod represents nothing...
Highlights from this year's European Stroke Organisation Conference (ESOC 2021; 1–3 September, virtual), Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT 2021; 8–10 September, Nice, France and virtual) and LINNC Paris Course (14–16 September, Paris, France and...
Imperative Care has announced that the first patient has been enrolled in the Imperative trial. This prospective, multicentre clinical trial is the first study designed to evaluate the clinical benefits of direct aspiration for the treatment of ischaemic stroke...
Outcomes in a cohort of patients who consented to one year of follow-up after transcarotid revascularisation (TCAR; Silk Road Medical) treatment for carotid stenosis in the ROADSTER 2 trial showed a 97.4% rate of technical success, no incidences of...
Avoiding a strategy of post-stent balloon dilation during transcarotid artery revascularisation (TCAR) may be unnecessary—a “contradistinction” to results shown in conventional transfemoral carotid artery stenting, new research has shown.
Jones Thomas, an integrated vascular surgery resident at University Hospitals Cleveland...
As evidence builds on the importance of reducing the risk of stroke by removing residual air prior to thoracic endovascular aortic repair (TEVAR) procedures, the onus will be on surgeons and device manufacturers to minimise this risk—as per a...
Medtronic has announced the publication of a study demonstrating, through the use of a continuous rhythm monitoring device, that atrial fibrillation (AF) is directly and transiently associated with ischaemic stroke. The findings were published online in JAMA Cardiology.
This retrospective...
The Wyss Center has announced CE mark Class I medical device approval for its Epios Cloud software—a web-based application for the online storage, processing and review of neural signals, including the ultra-long-term data recorded by an emerging generation of...
NeuroVasc Technologies has announced that the first patient has been treated in a pivotal randomised controlled trial (RCT) in China, which will evaluate the safety and efficacy of the ENVI-SR mechanical thrombectomy system (ENVI-SR)—a stent retriever for the removal...
A phase II, randomised clinical trial has found that the optimal period for intensive rehabilitation of arm and hand use after a stroke should begin 60 to 90 days after the event. The study, conducted by Georgetown University and...
Following the recent approval of the Senza system (Nevro) by the US Food and Drug Administration (FDA), Erika A Petersen (Little Rock, USA) discusses the impact of the only spinal cord stimulation (SCS) device indicated to treat chronic pain associated...
Medtronic today announced it has received CE mark approval for its radial artery access portfolio, which includes the Rist radial access selective catheter and the Rist 079 radial access guide catheter—the first catheter specifically designed for the unique demands of accessing...
Insightec has announced its incisionless neurosurgery platform, the Exablate 4000, has received market approval by the Central Drugs Standard Control Organization (CDSCO)—part of the Ministry of Health and Family Welfare of India.
The Exablate 4000 (Exablate Neuro) platform uses magnetic resonance imaging (MRI)-guided...
Medtronic has issued a recall of its Pipeline Flex embolisation device and Pipeline Flex embolisation device with Shield technology products due to a risk of the delivery system’s wire and tubes fracturing and breaking off during placement, retrieval, or...
This advertorial is sponsored by Boston Scientific and is only intended for readers in Europe.
Fast-acting sub-perception therapy (FAST™)—a novel innovation in the field of spinal cord stimulation (SCS), developed by Boston Scientific—is designed to deliver profound, paraesthesia-free pain relief...
Stroke care has seen considerable advances in recent years, and Stryker Neurovascular has played a role in these developments. In addition to expanding its portfolio to include cutting-edge products designed to remove blood clots in the brain quickly and...
NeuroOne Medical Technologies has announced the successful completion of an animal feasibility study of its combination recording and ablation technology at T3 Labs and Georgia State/Georgia Tech Center for Advanced Brain Imaging in Atlanta, USA under the guidance of...
At this year’s LINNC Paris Course (14–16 September, Paris, France and virtual), audiences heard that the ESCAPE-NEXT trial (sponsored by NoNO Inc.), which has already started enrolling patients and is expected to be completed by August 2022, could represent a major step...
Else Charlotte Sandset is a consultant neurologist in the Stroke Unit of the Department of Neurology at Oslo University Hospital, and chair of the Oslo Stroke Research Group (Oslo, Norway). Her research interests include blood pressure management in stroke...
Rhaeos has been awarded a US$4 million, multi-year grant from the National Institutes of Health (NIH) Small Business Innovation Research (SBIR) programme through the National Institute of Neurological Disorders and Stroke (NINDS). This funding will be used to expand...
Today, Boston Scientific announced an agreement to acquire Devoro Medical, developer of the Wolf thrombectomy platform. The non-console and lytic-free Wolf technology targets and captures blood clots using finger-like prongs that retrieve and remove thrombi in the arterial and...
This year’s Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT 2021; 8–10 September, Nice, France and virtual) saw details of the COATING (Coating to optimise aneurysm treatment in the new flow diverter generation; phenox) study presented...
Neuros Medical—a medical device company developing novel high-frequency nerve block technology for patients with intractable post-amputation pain—has announced the completion of enrolment in its pivotal QUEST (High-frequency nerve block for post-amputation pain) study.
QUEST is a 180-subject, randomised, double-blinded, active sham-controlled clinical...
A new study, published in the peer-reviewed medical journal Aging, marks the first time non-pharmaceutical clinical exploration has proved efficacious in reversing the main activators of Alzheimer’s disease. Using a specific protocol of hyperbaric oxygen therapy (HBOT), cerebral blood...
A novel augmented reality (AR) platform (Proximie) has been successfully used to teleproctor a neuroendovascular fellow during complex interventional and diagnostic procedures. That is according to a scientific abstract presented at the 13th Congress of the European Society of...
The CRISP (Comparison of paraesthesia mapping to anatomic midline-based burst programming strategies) study, published in Neuromodulation: Technology at the Neural Interface, has found that equivalent clinical benefits can be achieved in lead placement for burst spinal cord stimulation (SCS)...
Sensome has announced enrolment of the first patients in the multicentre, first-in-human CLOT OUT study, which will evaluate the safety and performance of the company’s Clotild smart guidewire system in large-vessel acute ischaemic stroke patients. The first patients were...
Highlights:
A closer look at the latest clinical data regarding flow diversion technologies – including new insights on the Flow redirection endoluminal device (FRED; MicroVention/Terumo) and five-year results from the SCENT trial (Stryker Neurovascular)
The unique ethical considerations created...
Highlights:
A closer look at the latest clinical data regarding flow diversion technologies - including new insights on the Flow redirection endoluminal device (FRED; MicroVention/Terumo) and five-year results from the SCENT trial (Stryker Neurovascular)
The unique ethical considerations created...
Transcarotid artery revascularisation (TCAR; Silk Road Medical) may be associated with favourable in-hospital outcomes for the treatment of restenotic carotid lesions after carotid endarterectomy (CEA)—following a study that also evaluated in-hospital outcomes of transfemoral carotid artery stenting (TFCAS) and...
Multiple pieces of clinical research supporting the use of flow diverter devices to treat intracranial aneurysms have come to light so far in 2021. In the past few weeks alone, data from a multicentre pivotal trial assessing the Flow...
“Like anything in the medical community, there can be positive and negative,” Agnieszka Solberg (Bismarck, USA) tells NeuroNews discussing the impact of social media in the fields of interventional and neurointerventional radiology.
As a founder of the RadChicks movement—an online...
Boston Scientific has announced the European launch of FAST—a new, fast-acting sub-perception therapy that the company says is clinically proven to demonstrate significant and sustained pain relief within minutes—for its WaveWriter Alpha spinal cord stimulator (SCS) systems.
While traditional paraesthesia-free...
Neuromod Devices has announced the appointment of Diarmuid Flavin as chief operating officer (COO). Flavin brings more than 25 years of global leadership experience in a range of medical device, technology and pharmaceutical companies to Neuromod, where he will...
The preliminary results of the SWIFT-DIRECT (Solitaire with the intention for thrombectomy plus IV t-PA versus direct Solitaire stent-retriever thrombectomy in acute anterior circulation stroke) trial have indicated that, in the treatment of large vessel occlusions (LVOs), direct mechanical...
Flying teams of interventionists directly to a primary stroke centre to perform endovascular therapy (EVT)—as opposed to waiting for the patient to be transferred to an intervention centre—may be associated with a higher probability of receiving EVT, a reduced...
NeuroOne Medical Technologies—a company focused on improving surgical care options and outcomes for patients with neurological disorders—has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market its Evo stereoelectroencephalography (SEEG) electrode technology for...
An exclusive interview covering a novel endovascular treatment option for communicating hydrocephalus (CH), the latest research involving deep brain stimulation (DBS) therapies, and data demonstrating the role mobile intervention teams could play in stroke care, all featured among the...
Artificial intelligence (AI) can be used to give stroke patients a personalised and more accurate assessment of their risk of suffering a recurrence, according to a new study presented at the 7th European Stroke Organisation Conference (ESOC 2021; 1–3...
Work stress, sleep disorders, and fatigue—which are regarded as non-traditional risk factors for heart attack and stroke—are rising more steeply among women than men, according to a new study presented at the 7th European Stroke Organisation Conference (ESOC 2021;...
MicroTransponder has announced US Food and Drug Administration (FDA) premarket approval of the Vivistim paired vagus nerve stimulation (VNS) system, which—according to a company press release—significantly improves the effectiveness of rehabilitation therapy for stroke survivors with moderate-to-severe upper extremity...
“We are starting to help patients in ways that we did not think were possible,” Thomas Oxley (Mount Sinai Hospital, New York, USA) tells NeuroNews, referring to the potential of brain-computer interface (BCI) technology.
Alongside his role as a vascular...
Carotid artery surgery and stenting have comparable long-term effects on fatal or disabling stroke in asymptomatic patients with severe carotid artery stenosis. That is the finding of late-breaking ACST-2 data presented in a Hot Line session at European Society of...
Presidio Medical has announced that research involving its novel ultra-low frequency (ULF) neuromodulation therapy has been published in Science Translational Medicine. The publication, titled “Neuromodulation using ultra low frequency current waveform reversibly blocks axonal conduction and chronic pain”, was...
Michael Fishman (Wilmington, USA) discusses the benefits of Differential Target Multiplexed spinal cord stimulation (DTM SCS; Medtronic)—when compared to more conventional SCS therapy options—and how this novel approach is driving an evolution in treatment paradigms for chronic low back...
Qure.ai has announced US Food and Drug Administration (FDA) 510(k) clearance for its brain computed tomography (CT) quantification product qER-Quant. Clinicians in the USA can now use this artificial intelligence (AI)-based tool to rapidly and precisely assess the severity...
Admitting adult craniotomy patients without significant comorbidities, who are expected to have a short length of stay, to the neuroscience ward has been associated with shorter stays and a reduced total cost of admission—with no significant differences in postoperative...
The National Institutes of Health (NIH) has collectively awarded Carnegie Mellon University, University of Pittsburgh Medical Center (UPMC), Mount Sinai Health System and Synchron with a US$10 million grant for the initiation of the US COMMAND clinical trial to...
SyntheticMR has entered a general software license agreement for distribution with Siemens Healthineers. The agreement enables Siemens Healthineers to market, sell and distribute SyntheticMR's product package, SyMRI Neuro—used to create quantitative magnetic resonance imaging (MRI) scans of the brain—to...
Rambam Healthcare Medical Center in Haifa, Israel is set to become the first medical centre in Israel to use virtual reality (VR) and augmented reality (AR) to plan and perform neurosurgeries—using technology provided by Surgical Theater. This extended reality...
Q'Apel Medical announced today the launch of the Armadillo radial access system—the latest application of its SelectFlex technology and part of the SelectFlex family of neurovascular access systems. Armadillo addresses the needs of both physicians and patients in a...
In May 2021, the American Heart Association (AHA)/American Stroke Association (ASA) updated one of its flagship guidelines—the secondary prevention of stroke guideline—for the first time since 2014, with the key takeaway being an emphasis on identifying the cause of...
In a randomised pilot study, temporary spinal cord stimulation (SCS) has demonstrated efficacy in the suppression of postoperative atrial fibrillation (AF) following coronary artery bypass graft (CABG) surgery, resulting in a lower burden of AF in comparison with patients receiving...
“I have tried several devices in the last 10 or 20 years, but I think this is absolutely different—this is the first time that we have treated non-vascular problems endovascular solution,” Pedro Lylyk (Clinica Sagrada Familia, Buenos Aires,...
Silk Road Medical has announced positive results from an independent analysis of standard surgical risk patients undergoing carotid endarterectomy (CEA) and transcarotid artery revascularisation (TCAR) for atherosclerotic carotid disease. The analysis was presented at the Society for Vascular Surgery’s...
Insightec has announced that Seoul National University Hospital (SNUH) is installing the company’s Exablate Neuro (Exablate 4000) platform—which uses focused ultrasound to ablate targets deep in the brain for a therapeutic effect.
"Focused Ultrasound provides a precision tool for neurosurgical...
William Mack is a professor of neurosurgery and vice-chair of academic affairs in the Department of Neurological Surgery at the University of Southern California (USC) Keck Medical Center, and the principal investigator and director of the Cerebrovascular Laboratory in...
A randomised controlled trial (RCT) involving 300 patients found that functional outcomes among basilar artery occlusion stroke patients “did not differ significantly” with endovascular therapy (EVT) compared to standard medical care. Nevertheless, writing in the New England Journal of...
Michael Chen, a neurointerventionist at Rush University Medical Center in Chicago, USA, has been named the president of the Society of NeuroInterventional Surgery (SNIS) and will now lead the society’s initiatives to advance excellence in minimally invasive neuroendovascular care,...
Fluid Biotech, a medical device start-up company based in Calgary, Canada, has announced the completion of an oversubscribed seed fundraising round totalling US$4.7 million—and the addition of two new members to its board of directors.
Fluid Biotech is commercialising what...
In a pilot programme in New York City, USA, a mobile interventional stroke team (MIST) travelled directly to patients to perform emergency stroke surgery instead of transferring stroke patients to a specialised stroke centre. This resulted in significantly less...
Flow Neuroscience, the creator of what is currently Europe’s only medically approved transcranial direct current stimulation (tDCS) device and behavioural therapy app for depression, has closed a US$9.3 million, oversubscribed Series A funding round led by Khosla Ventures, CSS...
The US Food and Drug Administration (FDA) has granted 510(k) approval for STAGE (Strategically acquired gradient echo), the latest magnetic resonance imaging (MRI) technology developed by SpinTech. STAGE is a post-processing software platform that enables comprehensive, quantitative brain imaging...
Following its acceptance into Frontiers in Neurology, Phenox has announced the results of a paper exploring the benefit of choosing a longer stentriever for mechanical thrombectomy procedures.
In a paper authored by Carmen Serna-Candel (Neuroradiologische Klinik, Klinikum Stuttgart, Stuttgart, Germany)...
While focused ultrasound is by no means a brand-new innovation in the field of neurotherapy—having first been deployed in the 1950s, if not earlier, for therapeutic, non-invasive purposes—the emergence of magnetic resonance imaging (MRI) guidance and other novel technologies...
The Flow Redirection Endoluminal Device stent system (FRED; MicroVention)—a flow diverter used to treat intracranial aneurysms—has been deemed safe and effective for this indication, following a multicentre pivotal trial designed to support an application for US Food and Drug...
A team of physicians at Allegheny Health Network (AHN) in Pittsburgh, USA has received US Food and Drug Administration (FDA) approval to begin the second phase of a “groundbreaking” clinical trial exploring the efficacy of deep brain stimulation (DBS)...
A new technology to help surgeons reduce the risk of aneurysm regrowth, Philips’ most recent contributions to the field of stroke care, and evidence on whether men and women respond differently to spinal cord stimulation (SCS), were of particular...
New findings published in Neurology, the medical journal of the American Academy of Neurology (AAN), have confirmed the long-term effectiveness of deep brain stimulation of the subthalamic nucleus (STN-DBS) in treating Parkinson’s disease—with a “significant improvement” in motor complications...
The Stentrode motor neuroprosthesis, developed by Synchron, has taken another step towards becoming the world’s first commercially available, endovascular brain-computer interface (BCI) technology after the company’s investigational device exemption (IDE) application received US Food and Drug Administration (FDA) approval....
Women may be less likely than men to be sent to a comprehensive, or “Level 1”, stroke centre for emergent large vessel occlusion (ELVO) ischaemic stroke. This gender disparity was highlighted in a study presented at the Society of NeuroInterventional...
At the Society of NeuroInterventional Surgery’s 18th annual meeting (SNIS; July 26–29 2021, Colorado Springs, USA and virtual), Jeffrey Saver, professor of Neurology at the University of California, Los Angeles (UCLA) Medical Center and director of the UCLA Stroke...
Balt today announced that it has completed enrolment in its FIRST study—an observational, prospective, multicentre, international, single-arm study aiming to collect safety and efficacy information on the use of the company’s Silk Vista and Silk Vista Baby flow diverters,...
Using a different type of computed tomography (CT) scan may reduce the time to essential surgery for patients with the deadliest kind of strokes—emergent large vessel occlusion (ELVO) ischaemic strokes. This is according to research presented at the Society...
A new tool for treating brain aneurysms may protect artery blood flow during surgery and reduce the risk of aneurysm regrowth, according to a study presented at the Society of NeuroInterventional Surgery’s 18th annual meeting (SNIS; July 26–29 2021,...
Black patients with stroke are less likely than white patients to undergo a potentially lifesaving, minimally-invasive thrombectomy procedure to remove blood clots from arteries in the brain, according to a study presented at the Society of NeuroInterventional Surgery’s 18th...
Neuro42, a US medical technology start-up focused on magnetic resonance imaging (MRI) and robotics, has announced the close of a US$6.5 million Series A financing round. Zepp Health led the financing round, and was joined by ShangBay Capital and...
Race, in combination with other factors, predicted how quickly individuals with stroke got to care centres to receive necessary neuroendovascular surgery, including thrombectomy, according to research presented at the Society of NeuroInterventional Surgery’s 18th annual meeting (SNIS; July 26–29...
Cerenovus, a neurovascular firm that forms part of The Johnson & Johnson Medical Devices Companies, will showcase its latest innovations in stroke care at the Society of NeuroInterventional Surgery annual meeting (SNIS; July 26–29 2021, Colorado Springs, USA and...
Peter A Schneider (San Francisco, USA) discusses the role stenting can play, alongside the existing best medical therapy, in treating asymptomatic carotid disease patients.
Best medical therapy (BMT) is mandatory in all patients with carotid occlusive disease. In addition, asymptomatic...
Omniscient Neurotechnology has received US Food and Drug Administration (FDA) 510(k) clearance for Quicktome—a digital brain-mapping platform that allows neurosurgeons to visualise and understand a patient's brain networks prior to performing life-changing brain surgery. In addition to FDA clearance...
Omniscient Neurotechnology, a Sydney-based brain-mapping technology company, has announced the completion of a AUD$40 million Series B financing round. The funding will allow Omniscient to accelerate its research and product portfolio, and expand its science, engineering, and sales teams...
Nevro has announced receipt of US Food and Drug Administration (FDA) approval for its Senza system for the treatment of chronic pain associated with painful diabetic neuropathy (PDN). According to a Nevro press release, this approval is specific to...
Highlights:
Insight on the benefits of endovascular therapy (EVT) for basilar artery occlusion from the BASICS (Basilar artery international cooperation study) trial
Why the latest update to one of the American Heart Association (AHA)’s flagship guidelines represents a “paradigm...
Highlights:
Insight on the benefits of endovascular therapy (EVT) for basilar artery occlusion from the BASICS (Basilar artery international cooperation study) trial
Why the latest update to one of the American Heart Association (AHA)'s flagship guidelines represents a "paradigm...
In a new scientific statement entitled “Primary Care of Adult Patients After Stroke”, the American Stroke Association (ASA)—a division of the American Heart Association (AHA)—has provided a roadmap for holistic, goal-directed and patient-centred primary care in the system for...
A systematic review and meta-analysis has demonstrated that patients undergoing carotid interventions after thrombolysis have a higher risk of periprocedural hazards, compared with those patients who did not have prior thrombolysis. Authors Stavros K Kakkos (University Hospital of Patras,...
Daniel Månsson (Flow Neuroscience, Malmö, Sweden) outlines the benefits held by transcranial direct-current stimulation (tDCS) in the treatment of depression—and how clinical evidence supporting its use for this and other mental health indications can be expanded even further in...
Brainomix has deployed its flagship artificial intelligence (AI)-based e-Stroke software across the majority of the largest comprehensive stroke centres in Poland, allowing the company to solidify its position as a European market leader in this space. The launch comes...
BlueWind Medical, a company making neuromodulation medical devices for the treatment of overactive bladder, has announced that the 100th patient has been enrolled in its OASIS (Overactive bladder stimulation system) study—a clinical pivotal trial designed to test the safety...
Kaneka Corporation has announced the signing of a sales alliance agreement with Asahi Intecc USA, through which—from August 2021 onwards—the company intends to further expand its sales in the US market.
This follows Kaneka gaining US Food and Drug Administration...
Repeated clot retrieval attempts are associated with an increased rate of emboli to new territory (ENT) and greater infarct growth—resulting in poorer functional outcomes in acute ischaemic stroke patients even after successful recanalisation has been achieved. These findings are...
Royal Philips has announced that the first patient has been enrolled in the WE-TRUST (Workflow optimisation to reduce time to endovascular reperfusion for ultra-fast stroke treatment) study at Vall d’Hebron University Hospital in Barcelona, Spain, marking the official start...
Robocath and Rennes University Hospital have announced they are launching a co-development research programme using robotics to improve treatments for stroke patients. With the support of Philips France, this unique programme will be implemented over the next four years.
It...
Insightec has announced approval of its Exablate Neuro platform by the Health Sciences Authority (HSA) in Singapore. The Exablate Neuro (Exablate 4000) platform uses focused ultrasound to ablate targets deep in the brain.
This unilateral treatment is suitable for patients...
In a study intended to explore gender differences in patients with failed back surgery syndrome (FBSS) or chronic visceral pain, men and women have been shown to respond equally well to spinal cord stimulation and dorsal root ganglion (DRG)...
Neuros Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) for the use of its novel, high-frequency nerve block system, Altius, as an aid in the management of chronic intractable pain of the lower...
Fresh evidence on the differences between directional and omnidirectional deep brain stimulation (DBS), as well as regulatory approvals for a new reperfusion catheter and an artificial intelligence (AI)-powered stroke guidewire, were among the most read stories featured on NeuroNews...
Viz.ai has been awarded a CE mark for its artificial intelligence (AI)-powered stroke care software, affirming its conformity with European health, safety and environmental protection standards for products sold within the European Economic Area. Viz.ai’s intelligent care coordination system...
Mainstay Medical has announced the limited US commercial launch of ReActiv8, its implantable restorative neurostimulation system to treat intractable chronic low back pain. The ReActiv8 system will be available through ReActiv8-certified physicians commencing in the summer of 2021.
“We are...
A study involving virtual participants—rather than real patients—was “as effective as traditional clinical trials” in evaluating the treatment of brain aneurysms using a flow diverter, according to new research. These findings, which are published in the journal Nature Communications,...
The Stroke Association has announced the top areas of research that need to be prioritised to improve stroke care. The announcement of these priorities—which have been set by stroke survivors, carers and professionals—marks the first UK-wide project to map...
Video retinal imaging company Epipole has announced it has begun working on a €1.1 million collaborative project to develop a technology solution for the diagnosis of intracranial pressure. The project is funded by the Eureka Eurostars programme, and is...
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance and commercial availability of the RED 62 reperfusion catheter—the latest addition to the company’s comprehensive Penumbra system. RED 62 is designed to navigate complex distal vessel anatomy and...
After speaking with experts, reviewing current evidence, surveying people living with stroke or a heart condition, and funding a telephone poll of Canadians, the Heart and Stroke Foundation of Canada has revealed it found there is “great promise, and...
Sensome has announced its Clotild Smart Guidewire System—designed to improve the treatment of ischaemic stroke patients—has been granted a Breakthrough Device designation by the Food and Drug Administration (FDA)’s Center for Devices and Radiological Health (CDRH).
“This is a tremendous...
Cambridge Consultants and Helius Medical Technologies have confirmed their “groundbreaking" Portable Neuromodulation Stimulator (PoNS) device—developed for the short-term treatment of gait deficit due to mild-to-moderate symptoms from multiple sclerosis (MS)—has received marketing authorisation from the US Food and Drug...
The endovascular treatment of intracranial aneurysms using the Pipeline Embolization Device (PED; Medtronic) has been deemed safe and effective following a study involving data on 1,000 aneurysms from a single-centre registry. The study, the findings of which are published...
Medtronic has received US Food and Drug Administration (FDA) approval for Vanta—a high-performance, recharge-free implantable neurostimulator (INS) with a device life that can be optimised up to 11 years. At comparable settings, the Vanta spinal cord stimulation platform offers...
Directional deep brain stimulation (DBS) has demonstrated benefits over omnidirectional DBS for the treatment of Parkinson’s disease in the largest prospective study in this space to date—with the former also being preferred by patients and clinicians alike. The findings...
Mitchell Elkind is a professor of Neurology and Epidemiology at Columbia University Irving Medical Center, and chief of the Division of Neurology Clinical Outcomes Research and Population Sciences (Neuro CORPS) in the Neurology Department of Columbia University, New York, USA. He is...
Medtronic has announced US Food and Drug Administration (FDA) approval and first US implants of the SenSight Directional Lead System, which is used for deep brain stimulation (DBS) therapy. SenSight is a first-of-its-kind DBS directional lead that combines...
By delivering small electrical pulses directly to the brain, deep brain stimulation (DBS) can ease tremors associated with Parkinson's disease or help relieve chronic pain. The technique works well for many patients, but researchers would like to make "next-generation"...
Vesalio has successfully secured a Class A financing round. The company claims this funding will be utilised to support its ongoing US Investigational Device Exemption (IDE) stroke study, establish US infrastructure, scale international business, and expand its innovative product...
Among people who have strokes and COVID-19, there is a higher incidence of severe stroke as well as a higher incidence of stroke in younger people, according to new data from a multinational group published in the journal Stroke....
New industry partnerships, the potential for energy savings in neuromodulation and the contentious topic of vertebral artery stenting were among the top stories that piqued the interest of NeuroNews' readers in May.
1. Medtronic, Viz.ai partner to bring “lifesaving” AI...
Medical imaging AI specialist Avicenna.AI has received CE mark certification for its CINA ASPECTS AI tool for stroke severity assessment. CINA ASPECTS automatically processes non-contrast computed tomography (CT) scans and calculates an Alberta Stroke Program Early CT (ASPECT) score...
A case series to assess transvenous paraspinal vein embolisation of cerebrospinal fluid (CSF)-venous fistulas—a novel treatment approach for spontaneous intracranial hypotension (SIH)—has resulted in a “clinical and radiographic improvement” across all the patients involved. The findings of this case...
RapidAI has announced the launch of its Rapid prehospital workflow solution for stroke, which optimises coordination between emergency medical services (EMS) providers and hospital care teams to help deliver patients the best care as fast as possible.
"Surviving a stroke...
Phil Taussky outlines a simple yet effective livestreaming platform that holds benefits for neurosurgeons during the ongoing COVID-19 crisis.
One of the challenges faced by the medical community during the COVID-19 pandemic has been how to continue training students, physicians,...
HeMo Bioengineering, a Singapore-based medical device company with a focus on treating stroke patients, has announced that its Afentta intracranial thrombectomy aspiration catheter—a product developed by the company’s branch in China—has received pre-market approval from the National Medical Products...
The European Union (EU) Medical Devices Regulation (MDR) takes effect from today (26 May 2021).
The Regulation revises quality and safety standards and the range of regulated devices and was first initiated in May 2017, with an initial three-year transition...
Identifying the cause of a stroke or transient ischaemic attack (TIA)—sometimes called a “mini-stroke”—can lead to specific prevention strategies to reduce the risk of additional strokes, according to updated guidelines from the American Stroke Association (ASA), a division of...
The noninferiority of mechanical thrombectomy alone—compared to mechanical thrombectomy plus intravenous tissue plasminogen activator (IV-tPA)—for the treatment of acute ischaemic stroke has been demonstrated across more than 109,000 patients within a national US database. These results were presented at...
The results of the HALO (High and low frequency options) study, which are published online in the scientific journal Neuromodulation: Technology at the Neural Interface, indicate that “major energy savings” are possible in the administration of subperception spinal cord...
Chordate Medical has received a CE marking that permits its Kinetic Oscillation Stimulation (KOS) technology to be marketed, sold and used for the treatment of chronic migraine within the European Union. This approval, which the company has described as...
Medtronic and Viz.ai have announced they are extending their successful US partnership to distribute Viz.ai’s "lifesaving" artificial intelligence (AI) software platform for stroke to Europe, the Middle East, and Africa (EMEA). The AI platform has been proven to synchronise...
The Society of Vascular and Interventional Neurology (SVIN) Mission Thrombectomy 2020+ Initiative (MT2020+) will announce the first annual World Stroke Thrombectomy Day at the Global MT Revolution Regional Conference on 15 May 2021. By designating this official day, MT2020+...
US medical device company Vesalio has announced the start of enrolment in the Vesalio CLEAR study—an acute ischaemic stroke FDA investigational device exemption (IDE) clinical trial to utilise its NeVa thrombectomy technology platform. Enrolment across 20 participating stroke treatment...
Psychiatric populations perceive the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) for mental health conditions to be beneficial, safe, and no more dangerous than conventional therapy options like medication—as indicated by a systematic review published in the peer-reviewed...
Neurovascular medical device company Perfuze has announced that the first five acute ischaemic stroke patients have been treated with its Millipede 088 clot aspiration catheter—having received CE mark approval for the device in February 2021.
“Our initial experience with the...
Perflow Medical has announced the first successful clinical use of its Stream17 Dynamic Neurothrombectomy Net—a lower profile device designed to effectively treat haemorrhagic and ischaemic stroke patients with more tortuous anatomies. The first clinical procedures using the device were...
Stenting is something that, for now at least, should be considered in the extracranial vertebral arteries only—and not the intracranial arteries—for symptomatic atherosclerotic stenosis, as indicated by a majority vote from attendees at the Charing Cross (CX) 2021 Digital...
The first US patients have been treated with the FDA-cleared TIGERTRIEVER revascularisation device (Rapid Medical). TIGERTRIEVER is the first physician-adjustable stent retriever, according to Rapid Medical, enabling neurointerventionists to better remove blood clots and restore blood flow to the...
The US Food and Drug Administration (FDA) has authorised marketing of a new device indicated for use in patients aged 18 and older undergoing stroke rehabilitation to facilitate muscle re-education, and for maintaining or increasing range of motion. The...
The early findings from a case report have highlighted the potential held by 3D black blood vessel wall post-contrast imaging to predict the outcomes of reperfusion therapy for distal vessel occlusion (DVO)—although the report’s authors also state that a...
Medtronic will partner with American health technology firm Surgical Theater to provide the first augmented reality (AR) platform for use in real time during complex cranial procedures. The partnership will see Medtronic's StealthStation S8 surgical navigation system interfaced with...
Leading UK charity the Stroke Association is funding what it claims will be the world’s first study to determine the long-term impact of COVID-19 on stroke survivors. The study will attempt to establish how differences in patients with and...
MindRhythm, a US medical technology company focused on preventing neurological injury in stroke, has announced the enrolment of the first patient in a multicentre trial. Through this trial, MindRhythm intends to demonstrate the effectiveness of its proprietary Harmony technology...
Thrombolysis is considered as effective as mechanical thrombectomy for the treatment of basilar artery occlusion (BAO)—despite existing evidence on both intervention options being limited. This is according to an audience poll conducted during the final day of the Charing Cross...
Medtronic has announced approval from the US Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate its internally-developed, implantable tibial neuromodulation (TNM) device, which is designed to provide relief from symptoms of...
Medtronic has received US Food and Drug Administration (FDA) approval for the Pipeline Flex embolization device with Shield Technology for the treatment of aneurysms.
Medtronic’s Shield Technology was developed to advance flow diversion therapy by introducing a surface-modified implant device...
Cerus Endovascular has announced that the US. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application to conduct a US trial for the Contour Neurovascular System™, indicated for the treatment of intracranial aneurysms.
The IDE approval...
A series of debates tested expert response to current acute stroke controversies during the final day of the Charing Cross (CX) 2021 Digital Edition (19–22 April, online). A major talking point at the Acute Stroke Controversies session was the...
This advertorial is sponsored by Medtronic
The field of neurointervention has seen a dramatic increase in the adoption of radial artery access over the past few years. In this article, Eric Peterson (University of Miami Miller School of Medicine, Miami,...
NovaSignal has announced the launch of the NovaGuide 2 platform, which provides clinical teams with real-time information about cerebral blood flow to guide diagnosis and improve patient outcomes.
According to a company press release, NovaGuide 2 is designed to seamlessly...
Moleac has announced the approval of the US Food and Drug Administration (FDA) of an investigational new drug (IND) application for MLC1501 in traumatic brain injury (TBI).
According to a company press release, Moleac is a biopharmaceutical company based in...
The Society of Neurointerventional Surgery (SNIS) and NeuroPoint Alliance (NPA) have announced an alliance between the Society of Vascular and Interventional Neurology (SVIN) and the NeuroVascular Quality Initiative-Quality Outcomes Database (NVQI-QOD).
NVQI-QOD is governed by the SNIS Patient Safety Organization...
During a presentation at the North American Neuromodulation Society (NANS) virtual meeting (15–16 January) entitled “Outcomes and Access to Neuromodulation for Women and Underrepresented Minorities: A Call to Action”, speakers discussed a range of factors that may contribute to...
In a late-breaking presentation given on the final day of the International Stroke Conference (ISC) virtual meeting (17–19 March), researchers presented a “promising” new thrombectomy device that is reported to achieve a successful reperfusion in 84.6% of patients.
Jeffrey Saver...
Highlights:
Coverage of the International Stroke Conference (ISC; 17–19 March, virtual), including results from the TIGER trial
The reasons women are still underrepresented in neurosurgery
A commentary on complete reperfusion versus near-complete reperfusion
Profile: Mitchell Elkind (New York, USA)
...
Highlights:
Coverage of the International Stroke Conference (ISC; 17–19 March, virtual), including results from the TIGER trial
The reasons women are still underrepresented in neurosurgery
A commentary on complete reperfusion versus near-complete reperfusion
Profile: Mitchell Elkind (New York, USA)
...
Oxford Endovascular has announced that it has raised US$10 million in a series A funding round for the development of its OxiFlow micro stent, an “origami” engineered medical device intended for the prevention of brain haemorrhage.
According to the company,...
Vysioneer, a provider of artificial intelligence (AI) for cancer care, has received clearance from the US Food and Drug Administration (FDA) for VBrain. In a press release, the company claims that this is the first-ever AI-powered tumour auto-contouring solution.
According...
A study published in the journal Neurology concludes that despite the reported stroke-related complications of COVID-19, there has been a global decline in the volume of stroke hospitalisations, the administration of intravenous thrombolysis (IVT), and inter-facility IVT transfers between primary and comprehensive...
Results of the BrainGate clinical trial, published in the journal IEEE Transactions on Biomedical Engineering, detail the first-in-human use of a new wireless brain-computer interface (BCI) in patients with tetraplegia. Researchers report that the new system is “functionally equivalent” to other wired systems.
According to researchers at Brown University...
Our top stories from March 2021, including: International Stroke Conference (ISC; 17–19 March, virtual) coverage, mechanical thrombectomy for posterior circulation distal, medium vessel occlusion (DMVO) found to be safe, why the neurosurgery field is male-dominated, and the US launch...
Irras has announced a strategic collaboration with the neurosurgical team at Aarhus University Hospital in Aarhus, Denmark, to evaluate the use of the Irraflow during the treatment of intraventricular haemorrhage (IVH) and assessment of brain compliance.
According to a press...
The use of transcranial magnetic stimulation (TMS) has a modest yet promising history in suicide reduction, according to the authors of a study published in Neuromodulation.
In this seed‐based functional connectivity analysis, Jennifer Barredo et al evaluated whether changes in...
Inbrain Neuroelectronics has announced that it has raised US$18.8 million in series A funding for its artificial intelligence (AI)-powered, graphene neuromodulation technology.
According to a company press release, Inbrain’s technology harnesses the power of graphene, a two-dimensional material first isolated in 2004...
Rapid Medical has announced the US Food and Drug Administration (FDA) clearance of its Tigertriever revascularisation device for use in the treatment of ischaemic stroke. According to a company press release, this is the world’s first adjustable thrombectomy device.
Rapid Medical claims...
Helius Medical Technologies has announced that its Portable Neuromodulation Stimulator (PoNS) device, for multiple sclerosis sufferers, has received marketing authorisation from the US Food and Drug Administration (FDA).
According to a company press release, the device is indicated for use as...
A recent Cerenovus study published in the Journal of Stroke (JOS) has found that in the treatment of acute ischaemic stroke, achieving complete or near complete reperfusion after a single attempt with mechanical thrombectomy (MT) not only provides improved functional...
The elevated risk of ischaemic stroke and transient ischaemic attack TIA in hospitalised COVID-19 patients may be lower than previously reported—although it is still higher than the risks associated with influenza and other infections, a late-breaking presentation at the...
A late-breaking science presentation at the International Stroke Conference (ISC) 2021 (17–19 March, online) reports that while the use of mechanical clot removal or endovascular thrombectomy (EVT) has increased, racial differences in treatment persist.
This study compared the use of...
Viz.ai presented new data supporting the use of its technology to coordinate care for acute ischaemic stroke, haemorrhagic stroke, and clinical trial recruitment at the International Stroke Conference (ISC) 2021 (17–19 March, virtual), according to a company press release.
Ameer...
A late-breaking abstract presented at the International Stroke Conference (ISC) 2021 (17–19 March, virtual) reports results from the MR CLEAN-NO IV trial, which is assessing the efficacy and safety of direct endovascular thrombectomy (EVT) compared with intravenous thrombolysis (IVT)...
Álvaro García-Tornel Garcia-Camba discusses his recent publication in Stroke, which aimed at evaluating the influence of time and collateral status on ischaemic core overestimation. This was a retrospective, single-centre study including patients with anterior circulation large-vessel stroke that achieved...
Medtronic has announced that it has received CE mark for the SenSight directional lead system for deep brain stimulation (DBS) therapy as treatment of symptoms associated with movement disorders and epilepsy.
According to a company press release, the SenSight directional...
Sevaro Health has announced the launch of Sevaro OneCall, a proprietary platform which, according to a company press release, enables immediate access to neurologists without the use of a call centre, now available as a separate offering or bundled...
A late-breaking abstract presented at the International Stroke Conference (ISC) 2021 (17 –19 March, online) has presented evidence that patients who received care from a mobile stroke unit (MSU) were less likely to have a disability three months after...
A late-breaking abstract presented at the International Stroke Conference (ISC) 2021 (17–19 March, online) reports that immediate angiography, rather than the standard computed tomography (CT scan), reduced stroke treatment time and was linked to improved recovery.
According to researchers, standard emergency department treatment for...
MagVenture has introduced the MagVenture Flow Arm — a patent-pending, clinical positioning system to optimise the delivery of MagVenture's FDA cleared protocols for non-invasive brain stimulation technology within the treatment of major depressive disorder and as an adjunct therapy...
BrainQ, an Israeli start-up with an artificial intelligence- (AI) powered therapeutic platform, has announced the results from a randomised controlled trial (RCT), showing 77% of treatment group recovered to a level of no symptoms or no significant disability on...
Factors including representation, a lack of mentoring, and discrimination have been among the issues cited by female neurosurgeons as contributing to gender disparity in the field. This is according to a study published in the Journal of Neurosurgery by Tina Lulla (Rutgers New Jersey Medical School,...
Preliminary research to be presented at the International Stroke Conference (ISC) 2021 meeting (17–19 March, virtual) has found that when NA1, a neuroprotectant, was delivered to the brain in nanoparticles, it reduced stroke severity and improved survival in a...
Preliminary research to be presented at the International Stroke Conference (ISC) 2021 meeting (17–19 March, virtual) has found that Black and Hispanic women aged 65–74 years old hospitalised with stroke had more severe strokes than their white counterparts.
According to...
Research to be presented at the International Stroke Conference (ISC) 2021 meeting (17-19 March, virtual) has found that for every 10-minute delay between arrival at the emergency room (ER) and starting stroke treatment, patients with severe strokes may lose...
Research to be presented at the International Stroke Conference (ISC) 2021 meeting (17–19 March, virtual) reports that neighbourhoods affected by the historic housing policies in the USA known as redlining were associated with higher rates of stroke.
Investigators report that...
Perflow Medical has launched its Cascade17 non-occlusive remodelling net to the European market. According to a company press release the device has already received its first clinical use.
The Cascade17 optimises non-occlusive support of distal and tortuous vessel anatomy during...
Healing Innovations has announced the completion of the first pilot for its new Rise&Walk InClinic technology at Shepherd Center in Atlanta, USA. The pilot included the technology's different use cases and gathered session data including clinician and patient feedback.
Healing...
Abbott has announced the US launch of its NeuroSphere Virtual Clinical, a first-of-its-kind technology that allows patients to communicate with physicians, ensure proper settings and functionality, and receive new treatment settings remotely as needed.
According to a press release from...
A study published in Stroke reviewing nearly 28,000 emergency department records across 54 healthcare facilities has shown that less than 2% of patients diagnosed with COVID-19 suffered an acute ischaemic stroke. However, the study also found that those who did suffer an ischaemic...
Our top stories from February 2021, including: The effectiveness of mobile stroke units, evidence for a new transient ischaemic attack (TIA) scoring system, endovascular thrombectomy (EVT) during the COVID-19 period, and commentary on complete versus near-complete reperfusion for acute...
Aesculap has announced its receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for DIR800 3D digital infrared fluorescence for use with its Aesculap Aeos robotic digital microscope. In a company press release, Aesculap report clearance...
Stratus Medical has announced that it has been granted a patent for its Nimbus radiofrequency (RF) multitined expandable electrode from the US Patent and Trademark Office. According to a company press release, its new patent contains 29 method claims, including claims...
A study recently published in The Journal of the American Medical Association (JAMA) has found that mechanical thrombectomy for posterior circulation distal, medium vessel occlusion (DMVO) is a reasonable, safe, and technically feasible therapy option.
The “Thrombectomy for Primary Distal Posterior...
Anuncia has announced that it has received US Food and Drug Administration (FDA) breakthrough device designation for its ReFlow system mini, intended for the treatment of cerebral spinal fluid (CSF) disorders requiring shunting.
Elsa Abruzzo, president of Anuncia, stated: "Our team is very pleased to achieve this...
Evasc Neurovascular has announced its successful application to France’s Ministry of Health (Haute Autorité de la Santé, HAS) Forfait Innovation (FI) programme for the eCLIPs device.
According to a company press release, the Forfait Innovation program grants clinical trial funding...
Results from a phase II clinical trial conducted by vascular neurologists at Cedar-Sinai, Los Angeles, USA, show there is potential to reduce the rate of recurrent stroke among atherosclerotic disease patients.
According to a press release from Cedar-Sinai, the phase...
Silk Road Medical has announced positive results from a comparative-effectiveness study have been published in JAMA Network Open.
The study found the availability of TCAR at a hospital was associated with a significant decrease in the likelihood of major adverse...
Ching-Jen Chen discusses his recent publication in the BMJ entitled, “Is a picture-perfect thrombectomy necessary in acute ischaemic stroke?”
Whether imaging findings translate into outcomes of clinical importance is often a point of contention in imaging-dependent medical specialties such as...
Newronika has announced the CE mark approval for its AlphaDBS system to treat Parkinson’s disease. According to a company press release, Newronika is a spin-off of the world-class neurological research centre Policlinico of Milan and University of Milan in Milan, Italy.
According to Newronika, AlphaDBS is the first rechargeable deep brain neurostimulation system that can continuously record...
Terumo Corporation has signed a definitive agreement to acquire all assets of Health Outcomes Sciences, a specialist in predictive analytics and clinical decision support for healthcare organisations.
“The acquisition of Health Outcomes Sciences and the ePRISM platform expands Terumo’s presence...
In a press release, icometrix announced the addition of icobrain cva, a stroke solution, to the icobrain portfolio. According to the company, this announcement follows clearance from the US Food and Drug Administration (FDA) and CE-marking of its image processing software for the analysis...
BrainStorm Cell Therapeutics announced it recently met with senior leadership from the US Food and Drug Administration (FDA) and received feedback on a high-level data summary from the NurOwn (autologous MSC-NTF cells) amyotrophic lateral sclerosis (ALS) phase III clinical trial.
According to a press release...
Stephanie Vanterpool (Knoxville, USA) talks to NeuroNews about how outreach can be used to address the disparities between white and underrepresented minority patients when it comes to accessing neuromodulation therapies.
A recent study, which looked at 1.2 million patients with...
“The most profound and scaring changes I felt are the impact of increased levels of anxiety and stress on the team,” comments Timo Krings regarding his learnings from COVID-19, which has had a considerable impact on his, and everyone...
Reporting a strong association between asymptomatic carotid stenosis and future stroke risk, Dominic Howard (Oxford University Hospitals NHS Trust, Oxford, UK) and colleagues write in The Lancet Neurology that their findings are “contrary to the assumptions of current guidelines...
Stryker has announced a new partnership with Brainomix that provides an option for European customers to purchase Brainomix’s e-Stroke software.
According to a press release from Stryker, the e-Stroke tool is an artificial intelligence (AI) powered software platform that provides...
In a study published in The Journal of the American Medical Association (JAMA), researchers have found that stroke patients in Berlin for whom STEMOs (Stroke-Einsatz-Mobile) were dispatched were more likely to survive without long-term disability.
According to Charité –...
phenox GmbH has announced the opening of a permanent UK office in the south of Birmingham, UK. The company states in a press release that this is in light of Brexit.
phenox GmbH states that its UK orders will now...
A study published in Neurorehabilitation and Neural Repair finds that the capacity of the human brain to recover and rewire itself peaks around two weeks after an ischaemic stroke, and diminishes over time.
These findings are the result of a...
Cerus Endovascular has received breakthrough device designation from the US Food and Drug Administration (FDA) for its Contour Neurovascular System.
The Contour Neurovascular System is indicated for the treatment of intracranial aneurysms. The company states that the product is composed...
A study recently published in the BMJ reports no observed delay in the treatment of patients with intracranial occlusion with endovascular thrombectomy (EVT) during the COVID-19 era when compared with the pre-COVID era.
The study is a retrospective review of observational...
A study published in the online issue of Neurology has found that people aged 70 and older are having fewer strokes and are also dying less frequently from strokes. In older people, researchers found declines in both ischaemic stroke...
CereVasc has announced the treatment of the first patient in a study of its eShunt System, an investigational device intended to treat communicating hydrocephalus (CH).
According to a company press release, this is the first minimally invasive treatment for CH....
ShiraTronics has announced that it has been granted a breakthrough device designation by the Center of Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA) for its neurostimulation therapy targeting chronic migraine sufferers.
The FDA breakthrough...
Azurity Pharmaceuticals has announced the purchase of Eton Pharmaceuticals' neurology portfolio.
These products include lamotrigine (ET-105), zonisamide (ET-104), and topiramate (ET-101). According to Azurity, these medications are currently under review with the US Food and Drug Administration (FDA) as new...
A new study published in the British Medical Journal (BMJ), has concluded that the Canadian transient ischaemic attack (TIA) score identifies the risk of patients with transient ischaemic attack (or mini-stroke) for a subsequent stroke or carotid artery revascularisation...
Vesalio has announced in a company press release that it has received 510(k) clearance from the US Food and Drug Administration (FDA) and its fourth CE mark.
According to Vesalio, the FDA 510(k) indication is for the removal of thrombi...
Boston Scientific has announced that it has received US Food and Drug Administration (FDA) approval for its fourth-generation Vercise Genus deep brain stimulation (DBS) system.
According to a company press release, the portfolio, approved for conditional use in a magnetic resonance imaging (MRI) environment, consists of a...
Biogen has announced that the US Food and Drug Administration (FDA) has approved a new intramuscular (IM) injection route of administration for Plegridy (peginterferon beta-1a) for the treatment of relapsing forms of multiple sclerosis (MS).
According to a company press...
Biomodex has announced its partnership with two of the top medical centres in France and a health system in the USA to develop Evias Plus, a training solution for treatment of ischaemic stroke.
“Thrombectomies are very complex in nature and...
Stuart Baker is a professor of Movement Neuroscience at Newcastle University, Newcastle, England. He previously presented virtually on this topic at the UK Stroke Forum, 7–9 December.
We all take the ability to move for granted, and most of the...
In a recommendation statement published on 2 February in The Journal of the American Medical Association (JAMA), the US Preventive Services Task Force (USPSTF) advises against screening for asymptomatic carotid artery stenosis in the general adult population. This recommendation...
Medtronic today announced expansion of the limited site release of its recently acquired radial artery access portfolio from privately-held Rist Neurovascular, following first-in-patient procedure. This portfolio includes the Rist 079 radial access guide catheter and Rist radial access selective...
Longeviti Neuro Solutions has announced the 510(k) clearance by the US Food and Drug Administration (FDA) of its ClearFit cranial implant, which it claims allows for ultrasound imaging post operation.
According to a company press release, the ClearFit implant is...
A new randomised controlled trial presented at the North American Neuromodulation Society (NANS) virtual meeting (15–16 January), presented by Robert E Gross (Emory University School of Medicine, Atlanta, USA), reports an average increase of 19% in word recall for...
At the North American Neuromodulation Society (NANS) virtual meeting (15–16 January), Dylan J Edwards, director of the Moss Rehabilitation Research Institute and director of Human Motor Recovery Laboratory (Philadelphia, USA), presented his proof-of-concept results for a study he led...
Results from a multi-centre, prospective, blinded, crossover study, presented at the North American Neuromodulation Society (NANS) virtual meeting (15–16 January) by Jan Vesper (Heinrich-Heine University, Düsseldorf, Germany) indicate better results from direction deep brain stimulation (DBS) in the subthalamic...
BioArctic AB has announced that the European Patent Office (EPO) has issued a decision to grant European patent EP 2 448 968 B1 for novel antibodies that could be developed into a treatment for Alzheimer's disease. The patent comes...
A late-breaking abstract presented at the North American Neuromodulation Society (NANS) 2021 virtual meeting (January 15–16) has shown that non-invasive neuromodulation may present a significant reduction in clinical opiate withdrawal scale (COWS) score.
The results were presented by Carlos...
CATHI has launched a campaign promoting CATHIS Smart, its portable system, to medical technology and pharmaceutical companies. CATHIS Smart displays all types of endovascular intervention in any medical field, and is particularly appropriate for neuroradiology, cardiology, radiology, angiology and...
The continued global burden of stroke and how it disproportionately affects women are highlighted in the February issue of Stroke, according to a press release from the American Heart Association (AHA).
Stroke editors selected nine manuscripts focused on stroke disparities in women...
Seneca Biopharma has announced its preliminary top-line results of the placebo-controlled phase II stroke study that was conducted in Beijing, China.
According to a company press release, the trial was designed to evaluate the relative safety of Seneca Biopharma's human...
New single-centre clinical study data published in the Journal of Neuroradiology claims to support the Anaconda Neurovascular Access (ANA) catheter system for the treatment of ischaemic stroke.
Investigators at Hospital Vall d’Hebron, Barcelona, Spain, are participating in the multi-centre SOLONDA...
VeraSci has announced a partnership with ActiGraph, to expand the use of wearables in clinical trials.
According to a company press release, VeraSci's Innovation Lab will be incorporating ActiGraph's CentrePoint Insight Watch into a National Institutes of Health (NIH) funded...
Highlights:
Coverage of the joint European Stroke Organisation and the World Stroke Organisation (WSO-ESO 2020) conference (7–9 November 2020), including how artificial intelligence can be used for automatic segmentation of stroke infarct volume
Exclusive interview with the leaders of...
Highlights:
Coverage of the joint European Stroke Organisation and the World Stroke Organisation (WSO-ESO 2020) conference (7–9 November 2020), including how artificial intelligence can be used for automatic segmentation of stroke infarct volume
Exclusive interview with the leaders of...
UCB has announced the launch of Nile AI, a separate company, with the aim of developing an epilepsy care management platform. According to UCB, the idea of the platform is to act as an extension of the healthcare provider...
Medtronic has announced the first enrolment in its trial evaluating the safety and efficacy of adaptive deep brain stimulation (aDBS) in patients with Parkinson’s disease (PD).
Medtronic reports that aDBS is an investigational feature of its Percept PC device, that...
The in silico trials for treatment of acute ischaemic stroke (INSIST) consortium began in 2017 and presented its concepts and first results at the joint European Stoke Organisation and World Stroke Organization (ESO-WSO) virtual conference (7–9 November). NeuroNews had...
Blanca Fuentes, María Alonso de Leciñana, and Exuperio Díez Tejedor write about the challenges faced in the management of stroke care throughout the pandemic, and the lessons learned. Fuentes originally presented on this topic at the joint European Stoke...
Mission Thrombectomy 2020+ (MT2020+), a global campaign of SVIN (Society of Vascular and Interventional Neurology), recently published Mechanical Thrombectomy for Acute Stroke. Building Thrombectomy Systems of Care in Your Region: Why and How—a white paper targeted towards health policymakers....
Pre-hospital stroke scales detect anterior circulation large vessel occlusion (aLVO) with acceptable-to-good accuracy, according to PRESTO, a large prospective observational study, the results of which were published in January 2021 in the Lancet.
PRESTO compared eight different pre-hospital stroke scales...
ClearMind Biomedical has announced 510(K) US Food and Drug Administration (FDA) clearance for its Axonpen System, which is used for the illumination and visualisation of intracranial tissue and fluids, and the controlled aspiration of tissue and/or fluid during surgery.
According...
Functional Neuromodulation has announced that the Vercise deep brain stimulation (DBS) device, developed and manufactured by Boston Scientific, has received breakthrough device designation from the US Food and Drug Administration (FDA). The device is aimed for treatment of patients...
BioSerenity has announced that its electroencephalography (EEG) wearable device system has received 510(k) clearance from the US Food and Drug Administration (FDA). This clearance includes BioSerenity’s Neuronaute EEG system and IceCap EEG wearable device, which are intended to allow...
Alnylam Pharmaceuticals has announced that the HELIOS-A phase III study of Vutrisiran, an investigational RNA interference (RNAi) therapeutic in development for the treatment of transthyretin-mediated (ATTR) amyloidosis, met its primary and both secondary endpoints at nine months in patients...
According to a press release by the American Academy of Neurology (AAN), a new study has found that while the prevalence of neurologic conditions like dementia, stroke, Parkinson's disease and multiple sclerosis (MS) is consistent across the USA, the...
A study published in the British Medical Journal (BMJ) has found that first-pass effect (FPE), restoring complete or near complete reperfusion (modified Thrombolysis in Cerebral Infarction 2c- 3) in a single pass, used as an endovascular treatment of...
Cerus Endovascular has announced in a press release the first-ever robotic-assisted intracranial implant of its Contour intrasaccular device.
The procedure was completed by Nitin Dange (King Edward Memorial Hospital, Mumbai, India) and was performed on a middle cerebral aneurysm with...
Rapid Medical has announced its Drivewire novel guidewire with a steerable distal top, has received US Food and Drug Administration (FDA) clearance. According to a company press release, Drivewire is the first neurovascular guidewire with a controllable distal end...
Cognoptix has announced new appointments to their senior management team. Carl Sadowsky (Nova SE University, West Palm Beach, USA) has been appointed as chief medical officer, and Mike Kaswan (New York, USA) as chief financial officer. Cognoptix is a...
This advertorial is sponsored by Medtronic Neurovascular
The deployment of an embolisation coil is “much more art than science” according to Daniel Sahlein (Goodman Campbell Brain and Spine, Indianapolis, USA). In this article, Sahlein considers the importance of embolisation coil...
Doctors at St George’s Hospital in London, UK are set to become the first within the UK National Health Service (NHS) to begin using Oxford Heartbeat’s PreSize Neurovascular technology following its receipt of CE-mark certification earlier this year.
A pilot study will...
A study published in Neurology claims that people hospitalised with COVID-19 and neurological problems including stroke and confusion, have a higher risk of dying than other COVID-19 patients. According to a press release by the Albert Einstein college of...
Emulate has unveiled a brain-chip aimed at enhancing neuroinflammatory disease research and drug discovery. According to a press release by emulate, the comprehensive neurovascular unit model emulates features of the human brain and blood-brain barrier.
Emulate claims the brain-chip is...
Biohaven Pharmaceutical Holding Company has announced that The Lancet has published the company's positive results from a phase III clinical trial of rimegepant for the preventive treatment of migraine in adults. According to a press release by the company,...
The US Food and Drug Administration (FDA) announced the urgent voluntary recall of Penumbra’s JET 7 catheters with Xtra Flex technology (JET 7 Xtra Flex) due to increased risk of mortality and serious injury. Penumbra initiated the recall on...
According to a press release produced by Theranica, a peer-reviewed study published in The Journal of Headache and Pain, has concluded that remote electrical neuromodulation (REN) was the only neuromodulation-based acute migraine treatment with sufficient clinical evidence to conclude...
Sandra Boccard-Binet is a research scientist at the University of Oxford, Oxford, UK. Her research focuses on deep brain stimulation. Here she writes about her personal experiences with transcranial Direct Current Stimulation (tDCS), an account which is currently in...
New research presented at the UK Stroke Forum (7–9 December, virtual) suggests that paramedics could use FaceTime to improve their assessment of someone experiencing stroke symptoms and help quickly and accurately identify stroke patients.1 This can ensure stroke patients...
Neurotrope announced the completion of an independent spin-off of neurotrope bioscience, which has now been renamed as Synaptogenix, trading under the symbol SNPX.
Daniel Alkon, the company’s chief science officer commented, "We expect Synaptogenix to benefit from the current environment...
Diadem presented its clinical Data and commercialisation plans for its AlzoSure prognostic biomarker test, a blood-based test for the early prediction of Alzheimer's disease (AD), at the 2020 Alzheimer’s Disease International Conference (ADI), December 2020. The company, reported these...
IRRAS AB has announced it has reached an exclusive distribution agreement for their IRRAflow device, with Anade SA, a well-established distributor of leading neurosurgical medical devices and technologies through Latin America, according to a press release by IRRAS.
In its...
Grant Mair, the Stroke Association’s Edith Murphy Foundation Senior Clinical Lecturer, University of Edinburgh, and honorary consultant neuroradiologist, NHS Lothian, Scotland. At the ESC-WSO 2020 conference he gave a talk entitled, “Large-scale clinically representative evaluation of e-ASPECTS & e-CTA...
Evasc has announced that the new Electric eCLIPs Bifurcation Remodeling System (eB) has been granted a CE Mark.
The eB builds on eCLIPs highly effective design, incorporating an improved delivery system with a torquable wire, a smaller size that is...
In a study published this week in Neuromodulation: Technology at the Neutral Interface, authors report that they have initial evidence that non-invasive vagal nerve stimulation (nVNS) is linked to better performance on visuospatial reasoning and memory recognition tasks.
This study...
Axonics, California, USA, has announced that a study, named ARTISAN, looking at its implantable sacral neuromodulation (SNM) devices for the treatment of urinary and bowel dysfunction, has provided some strong results for the Axonics r-SNM System.
The study was published...
NICO.LAB has received clearance from the US Food and Drug Administration (FDA) for its artificial intelligence (AI) powered solution in stroke care, StrokeViewer LVO.
According to NICO.LAB, StrokeViewer LVO is an AI algorithm for fast triaging of stroke patients. The...
In a new study published in the online issue of Neurology, researchers claim to have found difference in the left atrium in the hearts of black and white patients, which may play a role in the risk of stroke.
Hoomean...
Aviceanna.AI has announced that its medical imaging artificial intelligence (AI) tool, CINA Head, has qualified for the new technology add-on payment (NTAP) recently approved by the Centers for Medicare and Medicaid Services (CMS).
CINA Head analyses computer tomography angiogram (CTA)...
Heart & Stroke has announced that Alison Twiner has been appointed as a chair on its National Board of Directors. Twiner has served of Heart & Stroke’s National Board since 2014, and was most recently a Vice Chair. Her...
Wallaby Medical, a medical device company focused on stroke treatment, has announced the closing of a US$45 million Series C funding.
This round of funding was led by GL Ventures, the venture capital arm of Hillhouse Capital with participation from...
Rapid Medical has announced that its adjustable remodelling mesh device, called Comaneci, has received CE mark approval. Comaneci is intended to treat cerebral vasospasm by mechanically dilating the intracranial vessels.
According to Rapid Medical’s press release, this is the first...
The Society of Vascular and Interventional Neurology (SVIN) colleagues and James Siegler (Cooper University Hospital, Camden, USA) identify that it should come as no surprise that the COVID-19 pandemic and its many "waves" has led to significant paradigm shifts in...
Boston Scientific has announced that its Vercise Genus deep brain stimulation (DBS) device has received a CE Mark. This is a fourth generation device and has now been launched for a limited release onto the European Market.
The Vercise Genus...
The Stentrode brain-computer interface (BCI) has successfully been demonstrated by researchers to help patients with severe paralysis complete tasks such as texting, emailing, shopping and online banking, in a new study. The BCI does this without the need for...
The US Food and Drug Administration (FDA) has sent out a class one recall, the most serious level of recall, for Styrker’s Trevo XP ProVue retriever. This device is intended for the removal of blood clots during acute ischaemic...
A new study presented at the Radiological Society of North America (RSNA) annual meeting shows that COVID-19 patients with the pre-existing conditions hypertension and diabetes, were at an increased risk of complications such and bleeding in the brain and...
Fycompa or perampanel, an anti-epileptic agent, has now been approved by the European Commission (EC) for use in children, according to a press release by Eisai.
The drug has been approved for adjunctive treatment of partial-onset seizures with or without...
RapidAI, a neuroimaging stroke software platform which uses artificial intelligence (AI), has announced growth of nearly 300%. This has been attributed by RapidAI in a press release to an ”international adoption” of AI-powered stroke care.
RapidAI is approved for use...
Abbott has announced that their IonicRF, a minimally invasive radiofrequency ablation device, is now being launched in the USA. This is after the IonicRF device received clearance from the US Food and Drug Administration (FDA).
IonicRF is intended for the...
A study presented by Kelvin Wong (Houston Methodist, Houston, US) at the European Stroke Organisation and the World Stroke Organization joint virtual conference (ESO-WSO 2020, 7 –9 November, virtual), has shown that artificial intelligence (AI) can be used to...
Avicenna AI has announced they will partner with Canon Medical Systems to deliver a fully integrated stroke detection artificial intelligence (AI) solution.
Avicenna AI specialises in medical imaging AI, and has produced a programme, Cina Head triage AI solution for neurovascular emergencies, which has been...
A new report from the Stroke Alliance for Europe (SAFE), shows that if countries continue to fail to invest in stroke prevention, treatments and rehabilitation, the cost of stroke care across Europe could increase to €86 billion by 2040.
The study, named...
Results from a prespecified exploratory analysis of the positive THALES phase III trial have shown that for patients with acute ischaemic stroke or transient ischaemic attack (TIA), taking Brilinta (ticagrelor) 90mg twice daily along with aspirin for 30 days,...
A study presented by Johanna Ospel (University of Calgary, Calgary, Canada) at the European Stroke Organisation and the World Stroke Organization joint virtual conference (ESO-WSO 2020, 7-9 November, virtual), has shown that there is no reason to withhold endovascular...
The largest real world study of Phagenyx, a form of Pharyngeal Electrical Stimulation (PES), was published in the journal EClinicalMedicine – The Lancet, and showed significant benefits for those suffering from neurogenic dysphagia.
The study, the Pharyngeal electrical stimulation for...
A new survey from the Heart & Stroke Foundation of Canada, conducted on over 1200 people with conditions and their caregivers, found that approximately half of respondents rated virtual care as good as in person care. Eight in ten...
The pandemic has affected the lives of everyone across the world, but especially those in the medical field. Physician’s daily practices have changed, but the manufacturers of the medical products used every day have had to adapt, because a...
Salvia BioElectronics, Eindhoven, Netherlands, have received a Breakthrough Device Designation from the US Food and Drug Administration (FDA) for its implantable neurostimulation system for the treatment of drug resistant chronic migraine.
Wim Pollet, chief medical officer of Salvia BioElectronics stated,...
Alzinova AB, Stockholm, Sweden, have announced its upcoming clinical study for Alzheimer’s disease vaccine candidate ALZ-101, which will take place in the second quarter of 2021.
The subjects of this phase 1 study will be early Alzheimer’s disease patients. This...
Nurami Medical, a medical nanofiber technology company, has raised up to $6 million in series B funding to finance ongoing clinical trials for ArtiFascia. Funding will also be used for the regulatory approval process for the US Food and...
The organisers of the Live Interventional Neuroradiology, Neurology & Neurosurgery Course (LINNC) Paris 2020 event have announced its cancellation due to the French governments’ decision to impose a one month lockdown across Paris.
This was originally planned as a hybrid...
Medtronic has received their US Food and Drug (FDA) 530(k) clearance of their NIM Vital nerve monitoring system. The NIM system helps physicians identify, confirm, and monitor nerve function, helping to reduce the risk of nerve damage during head...
The society of Vascular and International Neurology (SVIN) has issued a white paper as part of their global campaign, Mission Thrombectomy 2020.
The paper, entitled ‘Mechanical Thrombectomy for Acute Stroke. Building Thrombectomy Systems of Care in Your Region: Why...
Virtual education offers unparalleled, and relatively inexpensive opportunities, but the joy of interacting in-person with colleagues and, frankly, appreciating a fine vintage after sessions, are both hard to replicate online, Jens Fiehler, professor and chairman, Diagnostic and Interventional Neuroradiology,...
Almost one in three of those who suffered a stroke during the pandemic delayed seeking medical attention due to COVID-19 fears. The Stroke Association highlight this due to World Stroke Day (today, 29 October).
The Stroke Association also highlight that...
A recent study, published by Neurology, found that p-tau T217 has better diagnostic accuracy for detecting Alzheimer’s disease than p-tau T181. Jozef Hanes, AXON Neuroscience R&D Services SE, Bratislava, Slovakia, and colleagues write that this higher level of accuracy...
Keck Medicine, of the University of Southern California, Los Angles, USA, is enrolling individuals in an international phase 3 clinical trial to study deep brain stimulation as a treatment for Alzheimer’s disease, sponsored by Functional Neuromodulation.
The clinical trial involves...
Neuromod has secured a series B €10.5 million financing for its Lenire tinnitus treatment device. This funding follows the publication of the company’s first large-scale clinical trial earlier this month.
This trial was conducted from 2016 to 2019 on 326...
NeoRhythm has been gathering customer feedback on its pulsed electromagnetic field therapy (PEMF) headband device, since its launch in 2019, to conclude the largest PEMF research to date.
Electromagnetic and bioelectromagnetic therapy pioneers Igor Jerman (BION Institute, Ljubljana,...
Medtronic has announced it has been implanted the first patient in its evaluation of InterStim Micro system performance and safety (ELITE) study of the InterStim Micro system.
The ELITE study is the only study of a rechargeable sacral neurostimulation (SNS)...
Mark H Paul, president of the Neurovascular division at Stryker, spoke to NeuroNews, for a launch of a new column titled MedTech Insights.
Paul, who has over three decades of experience in the “less invasive” medical device industry and...
In a session of the 2020 Cardiovascular and Radiological Society of Europe (CIRSE) meeting (12–15 September, virtual) focused on the hottest news in the endovascular thrombectomy space, Antonin Krajina (University Hospital, Hradec Králové, Czech Republic) shared new devices available...
Neuromod has announced the publication of the results of a clinical trial which has shown bimodal neuromodulation combining sound and electrical stimulation of the tongue can significantly reduce tinnitus symptoms.
The results of the TENT A1 (Treatment Evaluation of Neuromodulation for Tinnitus) trial,...
Cerenovus, part of Johnson & Johnson Medical Devices Companies, has today announced the European launch of Cerenovus Nimbus, a device designed to remove tough clots for successful revascularisation in patients with acute ischaemic stroke caused by a large vessel occlusion.
Ischaemic strokes account for 85% of all...
RapidAI states Rapid LVO is among the first software products to qualify for the New Technology Add-on Payment (NTAP).
NTAP is part of the CMS Inpatient Prospective Payment System (IPPS). A significant advancement in stroke care and reimbursement for Medicare...
A study published in the 30 September 2020 online issue of Neurology, the medical journal of the American Academy of Neurology, has found that stroke patients who get professional rehabilitation training through remote video consultations from their residence, may recover...
Biomarkers using mass cytometry can assess patient response to an emerging vaccine for a specific paediatric brain tumour, according to a recent multicentre study published in the Journal of Clinical Investigation.
Diffuse midline gliomas (DMG) and diffuse intrinsic pontine gliomas (DIPG) are...
Highlights:
Comprehensive coverage from the Society of NeuroInterventional Surgery (SNIS) 17th Annual meeting (4–7 August) including two new studies to describe outcome-related racial disparities among stroke patients
A first of its kind study has demonstrated the the potential for...
High-frequency optical coherence imaging can guide neurointerventional surgery to achieve optimal deployment of flow diverters, stents and intrasaccular devices, write Matthew Gounis and Ajit Puri. Here, they discuss the evolution of the technology, and detail their institution’s experience developing...
Stratus has secured two patents for its RF multitined expandable electrode, which the company says will protect the technology as procedural protocols shift from traditional radiofrequency ablation (RFA) needles to the Nimbus device.
The first patent granted, named System and...
Highlights:
Comprehensive coverage from the Society of NeuroInterventional Surgery (SNIS) 17th Annual meeting (4–7 August) including two new studies to describe outcome-related racial disparities among patients experiencing strokes
A first of its kind study has demonstrated the the potential...
A digital clock that sounds alarms signalling each step of acute stroke care at the hospital is a low cost tool that helped doctors in Germany streamline and accelerate the time-sensitive process, according to new research published recently in...
Azam Ahmed (Madison, USA; moderator) was joined by Demetrius Lopes (Chicago, USA) and Dan Gibson (Milwaukee, USA) as part of a special live NeuroNews webinar titled ‘Early adopters are changing the game in neurointerventional surgery’.
The webinar gave a global audience the chance to learn more about how three centres are utilising new functionalities of the ARTIS icono (Siemens Healthineers) angiography...
Personalised treatments for stroke and heart disease are closer thanks to ongoing charity funding for research at the University of Reading (Reading, UK).
The British Heart Foundation (BHF) has awarded Jon Gibbins and the team at the University of Reading’s...
Brainomix has announced the launch of the latest version of the company's stroke imaging platform, e-Stroke Suite 10.0, which includes clot detection from non-contrast computed tomography (CT) scans and is designed to enable clinicians to more broadly identify thrombectomy-eligible stroke patients.
The new software incorporates a series of artificial intelligence (AI) technologies, including...
Vesalio has announced it has expanded the distribution of the NeVa Thrombectomy System, gaining market coverage in 53 countries in Europe, Latin America, Asia and the Middle East.
Vice president of the company for international business, William von Brendel,...
Cerenovus recently announced that it has launched Stroke Solutions, which includes a suite of three devices designed to aid physicians in clot removal procedures. The announcement was made during the virtual European Society of Minimally Invasive Neurological Therapy (ESMINT;...
The last five years have seen endovascular therapy for patients with acute ischaemic stroke proven undeniably effective, changing the face of stroke treatment forever.
While the advent of mechanical thrombectomy has saved numerous lives and reduced disability in those that...
Virtual medical and rehabilitation appointments during COVID-19 could be paving the way to be a new norm, according to a recent review paper co-authored by Brodie Sakakibara (Kelowna, Canada) with the Centre for Chronic Disease Prevention and Management (CCDPM).
The paper, which...
The US Food and Drug Administration (FDA) has granted breakthrough device designation to the Stentrode brain-computer interface for its fully-implantable medical device that can translate brain activity or stimulate the nervous system from the inside of a blood vessel,...
A multicentre study has reported that clot perviousness or permeability, the ability for contrast used during the initial imaging workup to seep through a clot, as estimated by computed tomography (CT) imaging, is associated with ''first-pass success'' in large...
The Society of NeuroInterventional Surgery (SNIS) has announced that William Mack (Los Angeles, USA) has become the new president of the organisation. The announcement was made during the society’s’ 17th annual meeting (4–7 August), which was held virtually.
The change...
A new study released at the Society of NeuroInterventional Surgery's (SNIS) 17th Annual Meeting reveals that expanding standard techniques during mechanical thrombectomy, a procedure that removes a clot from an artery during stroke, allows researchers to reproducibly obtain and study...
Bilateral L4 dorsal root ganglion (DRG) stimulation has been shown to evoke strong and reproducible motor responses in the upper leg in patients with chronic motor complete spinal cord injury (SCI). In their paper published in Neuromodulation, authors Sadaf...
Methodist Le Bonheur Healthcare (MLH) is the first hospital system globally to participate in a landmark study using a first-of-its-kind EEG device.
A press release states that EEG (electroencephalogram) tests are conducted to detect problems in the brain that may...
RapidAI has announced that Rapid LVO has received US Food and Drug Administration (FDA) clearance for detecting suspected large vessel occlusions (LVOs).
Rapid LVO helps physicians speed up triage or transfer decision-making. The company state that the technology can work...
Wallaby Medical announced it has recently closed a series B+ financing round worth more than US$20 million. The company focuses on developing and commercialising medical device products for treating stroke. Wallaby Medical’s first product, the Avenir coil system, is...
Medtronic has announced that its recently US Food and Drug Administration (FDA)-approved InterStim Micro neurostimulator for sacral neuromodulation (SNM) therapy is now available in the USA. Cleveland Clinic (Cleveland, USA) performed the first patient implant in the nation with...
A new study has found several racial disparities after mechanical thrombectomy for stroke with respect to post-procedure management and outcomes. Vineeth Thirunavu of Northwestern University Feinberg School of Medicine, Chicago, USA, presented the data at the Society of NeuroInterventional...
Exposure to bridging therapy independently predicts haemorrhagic complications—without improving functional outcomes—in patients with atrial fibrillation (AF) associated stroke. The findings from this retrospective, registry analysis were presented by Feras Akbik (Emory University, Georgia, USA) during a late-breaking abstract session...
According to late-breaking data presented at the Society of NeuroInterventional Surgery’s (SNIS) 17th Annual Meeting, a wireless endovascular brain–computer interface (BCI) “may improve capacity to perform activities in daily living and functional independence” in patients with severe paralysis.
This conclusion was reached by Thomas Oxley (University...
Richard P Klucznik currently serves as the president of the Society of NeuroInterventional Surgery (SNIS) and the director of interventional neuroradiology at The Methodist Hospital (Houston, USA). Here, he talks to NeuroNews about his experience treating stroke throughout COVID-19,...
A survey of US neurointerventional non-physician procedural staff demonstrates a self-reported burnout prevalence of 51%. According to Patrick Brown of Wake Forest Baptist University (Winston-Salem, USA), who presented the data at the Society of NeuroInterventional Surgery’s (SNIS) 17th Annual...
The US Food and Drug Administration (FDA) has cleared the SOMATOM On.site, a mobile head computed tomography (CT) scanner from Siemens Healthineers that enables a critically ill patient to receive CT head imaging in the intensive care unit (ICU)...
Stryker announced that it has received US Food and Drug Administration (FDA) approval for an expanded indication of its Neuroform Atlas stent system as an adjunctive stent for use in the posterior circulation of the neurovasculature. In May 2019,...
From acute interventions to endovascular therapy indication expansion, Sunil Sheth from McGovern Medical School at UTHealth, Houston, USA, kick-started the Society of NeuroInterventional Surgery’s (SNIS) annual meeting (4–7 August) by outlining an array of recently completed, and ongoing stroke...
A new study presented at the Society of NeuroInterventional Surgery’s (SNIS) 17th Annual Meeting (4–7 August, virtual meeting) serves as the first prospective validation of the Rapid Arterial Occlusion Evaluation (RACE) scale in accurately identifying a large vessel occlusion...
Mortality rates after treatment of unruptured intracranial aneurysms have substantially decreased in the past decade, according to new findings presented at the Society of NeuroInterventional Surgery’s (SNIS) 17th Annual Meeting (4–7 August; virtual meeting).
The study sought to investigate trends...
Cerus Endovascular and AB Medica, a subsidiary of Balt, has announced that they have entered into a strategic distribution agreement providing AB Medica with the exclusive rights to market and sell the newly CE marked Contour Neurovascular System and...
Two-year results of Axonics’ ARTISAN-sacral neuromodulation (SNM) study demonstrate that patients implanted with the r-SNM system continue to receive clinically meaningful and statistically significant improvements in urinary urgency incontinence symptoms and quality of life.
The pivotal study was designed to...
Adding vagus nerve stimulation (VNS) therapy to treatment as usual has been demonstrated to improve outcomes in patients with treatment-resistant bipolar depression (TRBD). In a recent news release, LivaNova announces the publication of a new study in the International...
A novel tandem stent thrombectomy technique “seems to be a safe and effective” rescue treatment for acute large vessel occlusion (LVO) that is resistant to conventional attempts, concludes a study recently published in the Journal of NeuroInterventional Surgery.
The authors,...
Cerus Endovascular has announced that it has received CE mark approval for its CerusEndo MC 021 microcatheter, designed to allow physicians to access tortuous neurovasculature and deliver therapeutic devices to intended targets.
The CerusEndo MC 021 microcatheter, which already received...
Synchron has been awarded nearly AUD$1M ($990,000) in funding from the Medical Research Future Fund (MRFF), through MTPConnect, the Australian Government’s BioMedTech Horizons (BMTH) programme, to advance the commercialisation of Synchron’s Stentrode. Stentrode is a minimally-invasive brain-computer interface being...
Rapid Medical has announced CE mark approval for Tigertriever XL stentriever. The company noted that the first patients have been treated successfully with the neurovascular device.
According to the company, the device is the latest addition to the Tigertriever family...
Results from the positive phase III THALES trial showed AstraZeneca’s Brilinta (ticagrelor) 90mg used twice daily and taken with daily aspirin for 30 days, reduced the rate of the primary composite endpoint of stroke and death by 17% (HR...
Abbott announces it has received approval from the US Food and Drug Administration (FDA) for the use of its Patient Controller app on compatible personal Apple smartphone devices. According to Abbott, this approval allows patients living with neurological conditions,...
Highlights:
COVID-19 causes "widespread disruption" to neuroendovascular research around the globe
Stroke risk found higher in patients with COVID-19 than influenza
Thrombectomy proved efficacious in Latin America as the final results of RESILIENT are published
Profile: Richard P Klucznik...
Highlights:
COVID-19 causes "widespread disruption" to neuroendovascular research around the globe
Stroke risk found higher in patients with COVID-19 than influenza
Thrombectomy proved efficacious in Latin America as the final results of RESILIENT are published
Profile: Richard P Klucznik...
“Widespread disruption of neuroendovascular trials occurred because of COVID-19,” conclude the investigators of a survey-based study examining the impact of the pandemic. Now, as sites begin to resume clinical research, first author Ansaar T Rai of West Virginia University,...
Evidence in support of the motion that asleep surgery improves the therapeutic window for deep brain stimulation (DBS) when compared to awake surgery, has been published in the journal Neuromodulation. “Our study provides the first evidence for improvement of...
A higher rate of stroke has been reported in adults with COVID-19 compared to a cohort of patients with influenza. A retrospective study published in JAMA Neurology concludes that around 1.6% of adults with COVID-19 who visited the emergency...
Medical imaging artificial intelligence (AI) specialist Avicenna.AI has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA Head triage AI solution for neurovascular emergencies. The FDA’s decision covers CINA’s automatic detection...
According to a study published in Neuromodulation, five years after implantation, spinal cord stimulators have been explanted in 13.5% of patients. Furthermore, about 10% of these explants are for non-infections reasons. Predictors of non-infected explants include being younger, using...
An interventional clinical trial begins enrolling COVID-19 patients at Hospital Virgen del Carmen in Zarate, Argentina to evaluate the therapeutic effect of transcutaneous auricular vagus nerve stimulation (TAVNS) in patients with pneumonia associated with the disease. Hospital Virgen del...
A concerning new trend has been revealed by the Stroke Association. The charity states that the most at-risk groups are least likely to call for an ambulance due to not wanting to “burden the already busy emergency services”. These...
Balt has received CE mark approval for its Silk Vista flow diverting stent to treat patients with unruptured intracranial aneurysms, in turn enabling commercialisation in over 30 countries in and around the European Union.
Flow diverters are intended to divert...
Soterix Medical has announced that it received US Food and Drug Administration (FDA) investigational device exemption (IDE) to launch a trial of its non-invasive transcranial direct current simulation-limited total energy (tDCS-LTE) neuromodulation system. The system is an at-home platform intended...
LivaNova announces a publication in Contemporary Clinical Trials, which details the design for a prospective, multicentre, randomised controlled blinded trial demonstrating the safety and effectiveness of vagus nerve stimulation (VNS) therapy system as adjunctive therapy versus a no stimulation...
RapidAI has announced that Rapid ASPECTS has received US Food and Drug Administration (FDA) clearance as the first neuroimaging analysis device in the CADx (computer-assisted diagnostic software) category. Rapid ASPECTS is the only neuroimaging product shown to improve physicians’ interpretations of...
A study investigating chronic pain sufferers treated with burst spinal cord stimulation (B-SCS) has concluded that improvements were observed among all psychological measures at one year. The largest impact of the therapy was on catastrophizing and depression. “These pain-related...
“It is an amazing time to work in stroke medicine,” Jesse Dawson tells NeuroNews. Looking back at his career, he discusses the field’s biggest disappointment, and, in line with his own interests, believes the future of stroke treatment should...
Boston-based medical technology company Elucid Bio, maker of the Food and Drug Administration (FDA)-cleared and CE-marked vascuCAP software, announced Wednesday, July 1, that its novel artificial intelligence (AI) technology demonstrated a more than 70% improvement in accuracy of stroke...
Axonics, a company that has developed and is commercialising novel implantable sacral neuromodulation (SNM) devices for the treatment of urinary and bowel dysfunction, has announced that it has received US Food & Drug Administration (FDA) approval under a premarket...
The findings of the first clinical study to use the EVIAS (Biomodex) 3D printing models to plan aneurysm treatment using endovascular robotics and novel flow diverters have been published in the Journal of NeuroInterventional Surgery (JNIS).
The authors, Vitor Nagai...
Medtronic has announced it received US Food and Drug Administration (FDA) approval for the Percept PC deep brain stimulation (DBS) system. BrainSense technology makes Percept the first and only DBS neurostimulation system with the ability to chronically capture and...
Mainstay Medical has announced that the US Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for ReActiv8, its implantable neurostimulation system to treat intractable chronic low back pain.
“ReActiv8 is designed to be a restorative...
After receiving a standing ovation when presented at the European Stroke Organisation Conference (ESOC; 22–24 May, Milan, Italy) last year, the final results of RESILIENT have now been published in The New England Journal of Medicine.
The trial showed...
CereVasc, a company focused on the treatment of patients with hydrocephalus, announced the closing of an expanded Series A financing that raised US$43.9 million. The financing was led by the Perceptive Xontogeny Venture Fund and ATON Partners.
According to CereVasc,...
MIVI Neuroscience has announced today that the US Food and Drug Administration (FDA) has approved an investigational device exemption for the initiation of a human clinical study of the MIVI Q aspiration catheter in the USA. EVaQ is a...
Transradial, as opposed to transfemoral access, may be a safer approach for flow diversion procedures to treat cerebral aneurysms at a wide range of locations. This conclusion was reached by Yangchun Li from the University of Miami Miller School of Medicine (Miami, USA) and colleagues, as their retrospective...
Axonics, a company that has developed implantable sacral neuromodulation (SNM) devices for the treatment of urinary and bowel dysfunction, has announced US Food and Drug Administration (FDA) approval of its new wireless patient Remote Control with SmartMRI technology for...
phenox has announced the full European launch of the pRESET 5–40 and pRESET LUX 4–20 thrombectomy devices after receiving CE mark approval last year. The pRESET device is a stentriever which is used for mechanical thrombectomy in patients with...
A recently published study has shown fewer people than previously reported have had strokes as a result of COVID-19; however, strokes that accompany the novel coronavirus appear to be more severe. The analysis by Shadi Yaghi and colleagues from...
Adding vagus nerve stimulation (VNS) therapy to treatment as usual (TAU) has been demonstrated to improve outcomes in patients with treatment-resistant bipolar depression (TRBD). In a recent news release, LivaNova announces the publication of a new study in the...
Final results of the Basilar artery international cooperation study (BASICS) have been reported by Wouter J Schonewille (University Medical Centre Utrecht, Utrecht, the Netherlands) during a large clinical trials webinar hosted jointly by the European Stroke Organisation (ESO) and...
The final results of EFFECTS (Efficacy of fluoxetine: A randomised controlled trial in stroke) have been presented by Erik Lundström (Uppsala University, Uppsala, Sweden) during a large clinical trials webinar hosted jointly by the European Stroke Organisation (ESO) and...
Amid the COVID-19 pandemic, the need to prevent the spread of disease and manage critical hospital resources has never been more critical. In a letter to the editor of Neuromodulation, Philip Kim (Center for Interventional Pain Spine, Bryn Mawr,...
Balt has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to begin the Squid trial for the embolisation of the middle meningeal artery (STEM) for the treatment of chronic subdural haematoma.
A recent Veterans Affairs (VA) study of...
An article accepted into the journal Neuromodulation has concluded that, based on two case reports, non-invasive vagus nerve stimulation (nVNS) might provide clinical benefits in patients with COVID-19 via “multiple mechanisms”.
While most COVID-19 cases are mild, the authors,...
Investigators of the DIRECT-MT trial, who evaluated Chinese patients with acute ischaemic stroke from large-vessel occlusion, concluded that endovascular thrombectomy alone was non-inferior in terms of functional outcome, within a 20% margin of confidence, to endovascular thrombectomy preceded by...
Mayank Goyal and Johanna Ospel, both from the University of Calgary, Alberta, Canada, talk to NeuroNews about the challenges facing stroke care five years after endovascular therapy became the standard. But, as Goyal posits, “Right now, it is difficult...
New guidance from the American Academy of Neurology (AAN) recommends the closure of a patent foramen ovale (PFO) for some people who have had a stroke. The updated practice advisory has been published online in Neurology. Previous guidance concluded...
The association between COVID-19 and the increased incidence of stroke continues to grow. Situated within the pandemic’s epicentre, Kurt Yaeger and J Mocco, neurosurgeons at Mount Sinai Health System (New York, USA), discuss the unprecedented volume of stroke patients...
Highlights:
NeuroNews reports on a webinar hosted by SNIS that brought together physicians from Italy, China and the USA, each of whom detailed the lessons they have learnt from COVID-19 so far
Philip M Meyers and Arthur Wang describe...
Highlights:
NeuroNews reports on a webinar hosted by SNIS that brought together physicians from Italy, China and the USA, each detailing the lessons they have learnt from COVID-19 so far.
Philip M Meyers and Arthur Wang describe the state...
Prism Schneider (Cumming School of Medicine, University of Calgary, Calgary, Canada) and others write in a commentary in the Canadian Medical Association Journal—because of an increase in domestic violence during the pandemic—healthcare providers should be aware of the signs...
A spinal cord stimulation (SCS) training curriculum has been developed by a multidisciplinary taskforce of the North American Neuromodulation Society’s (NANS) education committee. It aims to standardise educational requirements across multiple training paths to SCS service, and defines Accreditation...
phenox has announced the European launch of the new p64 MW Flow modulation device, intended for the treatment of intracranial aneurysms. The device received CE mark in January 2020 and is available with the proprietary HPC (hydrophilic polymer coating)...
Cerus Endovascular has received CE Mark approval for its 0.021-inch Contour neurovascular system, compatible with smaller commercially available 021 microcatheters for the treatment of saccular intracranial aneurysms.
According to the company, the Contour neurovascular system is a fine mesh braid...
On 18 March, the Centers for Medicare & Medicaid Services (CMS) recommended “limiting non-essential care and expanding surge capacity into ambulatory surgical centres and other areas” to conserve resources and staff for managing COVID-19 patients. However, in a statement...
A case series of large-vessel stroke in patients younger than 50 years of age has been published in The New England Journal of Medicine. Authors, Thomas J Oxley and colleagues from Mount-Sinai Health System, New York, USA, write that...
“Telehealth is finally upon us. As someone who has been practicing in this world for several years, I was always waiting for an inflection point,” David Houghton, neurologist and chair of Telehealth and Digital Medicine at Oschner Health (New...
Cerus Endovascular has announced that it has received CE mark approval for its Neqstent Coil Assisted Flow Diverter device. The flow diverter is designed to treat intracranial aneurysms and a range of morphologies, including wide-necked bifurcation and bifurcation aneurysms....
With the original trial demonstrating a higher incidence of substantial reperfusion with tenecteplase compared with alteplase prior to endovascular therapy, EXTEND-IA TNK Part 2 sought to investigate the optimal dose. The investigators, Bruce CV Campbell and colleagues from Royal...
Following the announcement that the UK will remain on lockdown for the foreseeable future, NeuroNews speaks to Shahram Derakhshani about the impact of COVID-19 on stroke care in the country’s capital. “Society appears to be in shock,” he says,...
In a brief report in Annals of Internal Medicine, Seongman Bae (Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) and colleagues report that neither surgical nor cotton masks appear to be an effective approach for preventing the dissemination of SARS-CoV-2 from...
COVID-19 represents a global challenge that has exposed the USA’s fragmented public healthcare system, writes Demetrius Lopes. Here, he details Chicago’s action plan in response to the crisis, appropriately named, “Stroke Network on Steroids”, due to the speed and...
Axonics has announced the US Food and Drug Administration (FDA) approval of its next-generation rechargeable implantable neurostimulator for its r-SNM system under a premarket approval (PMA) supplement.
This next-generation implantable neurostimulator decreases how frequently a patient needs to recharge their implanted...
A webinar hosted by the Society of NeuroInterventional Surgery (SNIS) on 2 April brought together neurointerventionalists and endovascular neurosurgeons from Italy, China, and the USA. Important questions such as who to treat, whether to intubate, and how to optimise...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing sites in the USA.
Philips plans to double the production by May 2020 and achieve...
Medtronic has announced updates regarding its efforts to increase ventilator production around the globe. The company is announcing progress in the ramp-up of its ventilator production, as well as collaborating with technology partners and governments to drive new ventilator...
Medtronic has announced it is publicly sharing the design specifications for the Puritan Bennett 560 (PB 560) to enable participants across industries to evaluate options for rapid ventilator manufacturing to help doctors and patients dealing with COVID-19. This decision...
Stryker has announced the development of a limited-release emergency response bed to quickly aid healthcare providers with efficient care during the COVID-19 pandemic.
The Emergency Relief Bed is a low-cost solution intended to serve frontline healthcare providers. It includes a...
Rapid Medical has announced that it has completed enrolment in the TIGER (Treatment with Intent to generate reperfusion) study ahead of the planned scheduled. This is a USA-based, multicentre study of the performance of TIGERTRIEVER, the company's thrombectomy device...
Amid the chaos of the pandemic, it is becoming increasingly difficult, "if not impossible", to care both critically-ill COVID-19 sufferers and non-COVID patients simultaneously, Philip Meyers and Arthur Wang attest. Where minutes can mean the difference between life and...
The European Commission (EC) has adopted a proposal to postpone by one year the date of application of the new Medical Devices Regulation (MDR), which was due to come into force on 26 May this year. The postponement, a press...
A recent meta-analysis demonstrated that cervical non-invasive vagus nerve stimulation (nVNS) is a safe and effective method for acute pain relief in migraine and cluster headache. “Non-invasive VNS could be an alternative technique for patients unwilling to take their...
In a new statement published on 2 April 2020, all 45 societies represented by the US Council of Medical Specialty Societies (CMSS)—over 800,000 physicians—emphatically declare their belief that all frontline healthcare professionals must have access to personal protective equipment...
The Society of NeuroInterventional Surgery (SNIS) has published a set of recommendations for the care of emergent neurointerventional patients during the COVID-19 pandemic.
“Acute ischaemic stroke patients are a high-risk patient cohort,” the authors write, adding that patients with...
During a Q&A section of a European Commission (EC) college meeting on 25 March, EC spokesperson Stefan de Keersmaecker stated that the commission were looking to delay the “entry into force” of the new European medical device regulations (MDR) because of...
Ron Solar (ThermopeutiX, San Diego, USA) and colleagues report in EuroIntervention that the TwinFlo catheter (ThermopeutiX), in pigs, was associated with rapid, selective, deep cerebral hypothermia. They add that the catheter may “offer an improved method for neuroprotection during...
Diffusion Pharmaceuticals has announced that it expect delays in enrolment in the company’s ambulance-based PHAST-TSC phase 2 clinical trial in acute stroke due to the coronavirus. According to a news release, the LA County Fire Department informed the company...
A viewpoint in the Journal of the American Medical Association has offered potential solutions to modifying ongoing randomised clinical trials during the COVID-19 pandemic. It aims to “minimise disruption and preserve integrity”, while still ensuring participant health and safety.
Authors...
A novel intermittent dosing burst paradigm in spinal cord stimulation (SCS) has effectively relieved pain in patients with chronic intractable pain. Authors Timothy Deer, president and CEO of The Spine and Nerve Centre of the Virginias, Charleston, USA, and...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its response to the COVID-19 public health emergency in its day-to-day operations with industry.
The letter from...
Artio Medical has announced the recent presentation of interim nonclinical study results at the ABC-WIN Seminar in Val d'Isere, France. Presented by Blaise Baxter, a neurovascular and peripheral interventional radiologist, the interim data demonstrated that use of the Endura...
The American Heart Association (AHA) has committed US$2.5 million to research efforts to better understand the novel coronavirus, COVID-19, and its interaction with the body’s cardiovascular and cerebrovascular systems.
Specifically, the Association will be offering fast-tracked research grants for short-term...
A new Heart & Stroke guideline published today in the Canadian Medical Association Journal does not recommend taking ASA (Acetylsalicylic acid) as a preventive measure for those who do not have a history of stroke or heart or vascular disease,...
Having been honoured with the Best Basic Science Abstract Award at the North American Neuromodulation Society’s (NANS) 2020 annual meeting (23–26 January, Las Vegas, USA), Denise Wilkes now speaks to NeuroNews about how the perception of opioid treatment has...
NeuroPace has announced that its RNS System has received US Food and Drug Administration (FDA) approval of MRI labeling for the RNS System, expanding treatment options for the approximately one million patients in the USA living with seizures that...
Medtronic has issued an urgent field safety notice to warn on specific production lots of its Pipeline Flex embolisation devices due to the potential for device fracture.
The company initiated verbal communication about the issue last month after identifying the...
NeuroVasc Technologies has entered into a strategic partnership with the Wego Group that includes US$34 million in funding to support the company’s product portfolio development and global clinical trial programme.
With a focus on novel catheter-based technologies to treat neurovascular...
The final results of the ESCAPE-NA1 trial have been presented and published contemporaneously in The Lancet. The study investigated the safety and efficacy of nerinetide—a neuroprotectant drug—in subjects undergoing endovascular thrombectomy. At the International Stroke Conference (ISC; 19–21 February,...
LivaNova has announced a research collaboration with Verily, an Alphabet company, to capture measures of depression within its RECOVER clinical study, which is evaluating the effectiveness of vagus nerve stimulation therapy (VNS therapy) for difficult-to-treat depression (DTD).
Through this initial...
Treatment with tranexamic acid, a common medication that reduces bleeding, has led to a non-statistically significant reduction in intracerebral haemorrhage (ICH) growth, both in binary and absolute terms, according to data from the STOP-AUST trial presented during the late-breaking...
Cerus Endovascular receives US FDA approval for its first microcatheter. The 021 microcatheter will be offered with three different distal configurations, and commercial sales are expected to begin during the second quarter of 2020.
According to a press release, the...
Treatment with the blood thinner apixaban was associated with a lower risk of bleeding, death and hospitalisation compared with warfarin, regardless of history of prior stroke or blood clot. This secondary analysis of the AUGUSTUS trial was presented by...
Skipping IV thrombolysis and using mechanical clot removal alone for stroke was not shown to be non-inferior compared to the combination of both treatments, but was associated with a lower risk of intracerebral haemorrhage, according to late-breaking data presented...
Treating stroke patients in specialised ambulances speeds treatment and reduces patients’ disability, according to late-breaking science presented at the International Stroke Conference (ISC; 19–21 February, Los Angeles, USA).
Discussing the Berlin prehospital or usual delivery (B_PROUD) trial, Heinrich J Audebert,...
A brain stent appears safe and effective for reducing the risk of recurrent stroke in patients with cholesterol-clogged brain arteries, according to late-breaking data presented at the International Stroke Conference (ISC; 19–21 February, Los Angeles, USA).
Discussing the one-year results...
A new, non-invasive wearable magnetic brain stimulation device to improve recovery for stroke survivors has been deemed safe and effective. David Chiu, director of the Eddy Scurlock Stroke Center at Houston Methodist Hospital, Houston, USA, presented the preliminary late-breaking...
Current direct endovascular therapy access for acute ischaemic stroke within 15 minutes is limited to one-fifth of the US population, concludes a recent study presented by Amrou Sarraj at the International Stroke Conference (ISC; 19–21 February, Los Angeles,...
Viz.ai has launched the next generation of its synchronized care platform for stroke. Viz.ai incorporates cutting edge artificial intelligence with HIPAA-compliant communication to allow stroke teams to quickly and efficiently triage patients to time-sensitive care with the aim of...
Both Blacks and Hispanics of Caribbean descent living in Northern Manhattan have a significantly higher risk of stroke than their non-Hispanic, white neighbours, according to preliminary research to be presented today at the International Stroke Conference (ISC; 19–21 February,...
Cerus Endovascular has received CE mark approval for its lead product, the Contour Neurovascular System, for the treatment of intracranial aneurysms. The system incorporates a fine mesh braid that is deployed across the neck of the aneurysm sac and...
A portable, low-field magnetic resonance imaging (MRI) system may become a safe and practical way to get accurate brain images at a patient’s bedside, according to preliminary research to be presented at the American Stroke Association’s International Stroke Conference...
A randomised, double-blind trial finds ECAP-controlled closed-loop spinal cord stimulation (SCS) statistically superior for the treatment of chronic pain, compared to open-loop stimulation. At 12-months, >83% of closed-loop patients reached the ≥50% responder threshold and >56% reached the ≥80%...
Rist Neurovascular recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market the Rist Cath Radial Access Long Sheath (Rist Cath) for the introduction of interventional devices into the peripheral, coronary, and neurovascular...
Jason Pope is the president and CEO of Evolve Restorative Center in Santa Rosa, USA, and serves as president of the Californian Society of Interventional Pain Physicians. Here, he talks to NeuroNews about how he has seen the field...
A multicentre study across 18 centres finds 10kHz safe and effective for the treatment of painful diabetic neuropathy. Sensory improvements were observed in many patients who underwent stimulation with these parameters, as well improvements in quality of life measures....
Highlights:
NeuroNews exclusively interviews Vitor Mendes Pereira (Toronto, Canada) who has recently performed the world’s first-in-human robotic-assisted brain surgery
Demetrius Lopes (Chicago, USA) discusses his work on the surface modification of stents and flow retrievers
Profile: Jason Pope (Santa...
Highlights:
NeuroNews exclusively interviews Vitor Mendes Pereira (Toronto, Canada) who has recently performed the world’s first-in-human robotic-assisted brain surgery
Demetrius Lopes (Chicago, USA) discusses his work on the surface modification of stents and flow retrievers
Profile: Jason Pope (Santa...
Stroke treatment is time-sensitive. Every minute counts. Every delay in decision-making affects patient outcome. Given this, JM Ospel and colleagues from the University of Calgary, Canada, write that it is imperative that stroke physicians are able to quickly and...
Axonics announces US Food and Drug Administration (FDA) approval of an enhanced, second-generation programmer for its r-SNM system under a premarket approval (PMA) application supplement.
The new programmer is used to program the Axonics external trial neurostimulator as well...
Stratus Medical, a new company created to focus on advancing radiofrequency (RF) ablation treatment for chronic pain, has announced that it completed a private placement to acquire the assets of NimbusRF from Biomerics and provide growth capital funding to...
A study investigating the relationship between systolic blood pressure (BP) reduction and outcome of mechanical thrombectomy has shown that systolic BP, in the first 24 hours after successful reperfusion, is inversely associated with poor outcome. Regardless, the authors, Mohammad...
Data showing the effective use of a long-term vagus nerve implant in mice have been presented at the North American Neuromodulation Society’s (NANS) annual meeting (23–26 January, Las Vegas, USA). Ibrahim Mughrabi from The Feinstein Institutes for Medical Research...
While recognising that excellence derives from experience, Robert M Levy suggests that the neuromodulation community can better protect the field’s “desire to ensure excellence for patients and healthcare economies”. Here, he makes a case for establishing centres of excellence...
Medtronic announce the launch of Efficio, a cloud-based data management software for use with the SynchroMed II intrathecal drug delivery system, that will allow clinicians to more efficiently manage their targeted drug delivery pump practices to treat patients with...
Three-month results from a large, multicentre randomised controlled trial (RCT) has demonstrated statistically significant (p=0.0009) and superior back pain relief with differential target multiplexed (DTM) spinal cord stimulation (SCS) compared to conventional SCS.
With both arms using the Intellis...
Abbott has announced it received approval from the US Food and Drug Administration (FDA) for a new, expanded indication for the company's Infinity deep brain stimulation (DBS) system to include targeting of an area of the brain called the...
New quality of life and functional improvement data from both the BOLD and TRIUMP studies echo the benefits of the Proclaim XR neurostimulation system (Abbott) for the treatment of chronic pain. Presenting the results at the North American Neuromodulation...
Data from a prospective, multicentre trial examining high-frequency spinal cord stimulation (SCS) at 10kHz for the treatment of chronic pelvic pain supports the safety and effectiveness of the therapy. “A clinically meaningful improvement for pelvic pain was indicated through...
SPR Therapeutics has announced publication of a prospective case series regarding its SPRINT peripheral nerve stimulation (PNS) system in the treatment of low back pain (LBP) demonstrating sustained pain relief at one year following treatment in the majority of...
Results of the first-in-human analysis of acute interactions and healing process after flow diverter implantation using optical coherence tomography (OCT) imaging was presented by Boris Pabón and Juan Mejia as members of Angioteam, Angiodinamia, Clinica Del Norte, Medellín, Colombia,...
Medtronic has announced it has received CE mark for its InterStim Micro neurostimulator and InterStim SureScan MRI leads—clearing the technologies for commercial sale and clinical use in Europe. The availability of the new technologies on 15 January will expand...
Aidoc has announced that the US Food and Drug Administration (FDA) has cleared its AI solution for flagging large vessel occlusion (LVO) in head CTA scans, marking Aidoc's fourth FDA-cleared AI package. Combined with Aidoc's previously-cleared AI module for...
The preliminary safety data of a “self-expanding primarily bioabsorbable flow-diverting” stent has shown to be consistent with a favourable profile in terms of mechanical behaviour, haemocompatibility, side branch patency, and histological effects. Intended for aneurysm use, the authors write...
MicroVention, a US based subsidiary of Terumo and a global neurovascular company announced the US FDA premarket approval (PMA) for the FRED (Flow re-direction endoluminal device) device for the treatment of brain aneurysms.
The FRED device uses a self-expanding braided...
Medtronic announces the CE mark for Percept PC neurostimulator. It is the only Deep Brain Stimulation (DBS) system to be launched in the European Union (EU) with BrainSense technology that can sense and record brain signals while delivering therapy...
Medtronic has acquired Stimgenics, a privately-held company that has pioneered a novel spinal cord stimulation (SCS) waveform known as Differential Target Multiplexed (DTM) Spinal Cord Stimulation. The therapy, which is delivered via the Medtronic Intellis platform, is a new...
MicroVention, a US-based subsidiary of Terumo and a global neurovascular company, announces the US FDA premarket approval (PMA) for the WEB aneurysm embolisation system for the treatment of intracranial wide neck bifurcation aneurysms. The WEB system is the first...
Demetrius Lopes has been working on the surface modification of stents and flow diverters, given that thromboembolic and antiplatelet-related complications represent the majority of those reported when using these devices. His aim is to decrease their thrombogenicity and mitigate...
Saluda Medical has announced The Lancet Neurology journal published results from the US pivotal study demonstrating its Evoke ECAP-controlled, closed-loop spinal cord stimulation (SCS) system provided long-term, statistically superior, and clinically meaningful pain relief for patients with chronic intractable...
Axonics has announced that the United States Patent and Trademark Office has issued or allowed Axonics nine US utility patents in 2019, along with numerous foreign counterparts relating to its implantable sacral neuromodulation technology.
The US patents range in subject...
Aleva Neurotherapeutics has announced that it has been awarded the CE mark for its flagship product, the directSTIM Deep Brain Stimulation System. The system incorporates directional electrode technology and is designed to be more precise and efficient, with optimised...
Imperative Care has completed the initial closing of a Series C financing of US$85 million to support the commercial launch of the company’s portfolio, which is composed of the latest advances in neurovascular devices treating ischaemic and haemorrhagic strokes.
The...
Axonics, a medical technology company that has developed and is commercialising novel implantable Sacral Neuromodulation (SNM) devices for the treatment of bladder and bowel dysfunction, announces completion of a post-approval US physician seminar series and the results of surveys...
The first European study to evaluate the economic impact of first-pass effect (FPE) found that FPE patients not only benefit clinically, they also endorse favourable economic outcomes. At the 15th Congress of the World Federation of Interventional and Therapeutic...
The first educational module for neuromodulation, a masters-level e-learning course aimed at nurses and allied healthcare professionals working within the field, received its first cohort of students in September of 2019. However, the story of its launch is not...
Philips announces the launch of IntelliSpace AI Workflow Suite to enable healthcare providers to seamlessly integrate AI applications into the imaging workflow. Part of Philips’ new enterprise imaging informatics solution, the comprehensive AI workflow platform provides a full suite...
NeuroPace has announced that new data from a multicentre, real-world clinical study of the RNS system has demonstrated outcomes never before seen with other neuromodulation therapies for focal epilepsy. Data from this study and more than 30 abstracts related...
Philips has announced a major clinical trial to assess the impact of a direct-to-angiosuite workflow on stroke patient outcomes. The study will assess whether with Philips’ advanced image-guided therapy platform it is possible to diagnose, plan and treat stroke...
Dorsal root ganglion (DRG) stimulation is a safe and effective therapy for multiple chronic pain disorders, concludes a pooled analysis published in Neuromodulation. The authors, Frank J P M Huygen (Erasmus University Medical Center, Rotterdam, the Netherlands) and colleagues...
Viz.ai has announced a number of new features at the Society of Vascular and Interventional Neurology (SVIN) annual meeting in Atlanta, USA (20–23 November, 2019). The latest software, available as a free software update to Viz.ai customers, features customisable...
A recently published systematic review reveals that artificial intelligence (AI) is rapidly being used by major medical centres to identify large vessel occlusions (LVO) and diagnose stroke. While AI has the potential to expedite treatment and address a critical...
Since founding the Ghana Society of Interventional Radiologists in November 2015, Benjamin Dabo Sarkodie has been working to establish the offering of minimally invasive, image-guided procedures in the west African nation. He describes his recent successes performing the first...
Axonics, a medical technology company that has developed and is commercialising novel implantable rechargeable sacral neuromodulation (SNM) devices for the treatment of bladder and bowel dysfunction, has announced the approval of the Axonics r-SNM system by the US Food...
On the 8th of November 2019, during a symposium at the annual meeting of the Hungarian Neuroradiology Society (MNRT) in Mátraháza, Hungary, Bence Gunda shared results from his hospital which showed that treatment rates for stroke patients improved and...
Nick Hopkins (Buffalo, USA) talks to BLearning Neuro at VEITH Symposium 2018 (13–17 November, New York, USA) about the DAWN trial, which showed that, in carefully selected acute ischaemic stroke patients, “intervention out to 24 hours is viable”.
“Stroke intervention is...
The recent European Society of Minimally Invasive Neurological Therapy (ESMINT; 4–6 September, Nice, France) meeting saw Adriaan C G M van Es of Erasmus Medical Center in Rotterdam, the Netherlands, present an update on the MR CLEAN II trials...
Researchers at The Feinstein Institutes for Medical Research used new closed-loop neurostimulation methods and textile-based electrodes to facilitate individual finger movement and grasp force regulation in quadriplegia individuals. Their results were published in Springer Nature, Bioelectronic Medicine.
Approximately 5.4 million...
The first-in-human robotic-assisted neurovascular intervention has been performed at the Toronto Western Hospital (Toronto, Canada), by a team led by Vitor Mendes Pereira. A press release states that this “landmark” robotic-assisted stent-assisted aneurysm coiling case marks a “significant milestone”...
Mario Muto (Naples, Italy), president of WFITN 2019 (World Federation of Interventional and Therapeutic Neuroradiology; 21-24 October; Naples, Italy), talks to BLearning Neuro about challenges in the neuroradiology field.
Muto says that while endovascular, minimally invasive treatments being offered to treat stroke...
Joanna Schaafsma (Toronto, Canada) talks to BLearning Neuro at WFITN 2019 (World Federation of Interventional and Therapeutic Neuroradiology; 21–24 October; Naples, Italy) about the potential benefits and downsides of intracranial vessel wall imaging in stroke patients. Intracranial vessel wall imaging “mostly benefits”...
Nevro today announced it has received approval from the US Food and Drug Administration (FDA) for the Senza Omnia spinal cord stimulation (SCS) system. The Omnia system is the first and only SCS system designed to deliver Nevro's proprietary...
Axonics has announced the first US-based implantation of the Axonics r-SNM system subsequent to its clearance by the US Food & Drug Administration (FDA) in September 2019. Following the completion of customary post-approval regulatory activities, the first implant outside...
Thrombolytic Science (TSI) has announced progress with 10 patients enrolled in its prospective, randomised, multicentre phase 2 clinical trial, designed to confirm the safety and efficacy of the sequential dual-treatment regimen of low-dose tissue plasminogen activator (tPA) and HisproUK...
“Parent vessel-preserving strategies cannot be considered inferior to parent vessel occlusion ,” concluded Gopinathan Anil, National University Hospital, Singapore, during his presentation on dissecting distal cerebellar artery aneurysms at the recent World Federation of Interventional and Therapeutic Neuroradiology (WFITN)...
Waleed Brinjikji talks to BLearning Neuro at WFITN 2019 (World Federation of Interventional and Therapeutic Neuroradiology; 21–24 October, Naples, Italy) about his presentation on selective brain cooling, a process where the internal carotid artery or the middle cerebral artery are cooled by either...
GTX Medical (formerly G-Therapeutics) and NeuroRecovery Technologies (NRT) have announced a merger, creating a global company for the development of neuromodulation therapies to aid functional recovery of people with spinal cord injuries. The merged entity will be known as...
Boston Scientific has announced the CE mark approval of the Vercise Neural Navigator 3 directional deep brain stimulation (DBS) programming software—the first system that integrates patient-specific 3D brain anatomy and stimulation field modelling into the clinician’s programming interface. When...
Perflow Medical has announced they have received CE Mark approval for the Cascade Agile non-occlusive remodelling net (Cascade Agile). Expanding the Cascade product family, the Cascade Agile optimises control for distal and tortuous vessel anatomy during coil embolisation of...
iSchemaView has launched the newest addition to the RAPID platform, RAPID ICH at the 2019 Congress of Neurological Surgeons (CNS) Annual Meeting (19–23 October, San Francisco, USA). This fully-automated system uses the latest in artificial intelligence to quickly triage...
Synchron recently announced the first successful implant of its minimally-invasive neural interface technology (Stentrode) as part of a trial evaluating the safety and efficacy of the technology for restoring communication in people with severe paralysis. The technology uses modular training...
Imperative Care has announced that Brian Armijo has been appointed as vice president of operations. With more than 25 years of manufacturing and operations experience and leadership and an extensive background in the cardiovascular space, Armijo brings significant relevant...
For patients with emergent large vessel occlusion (LVO) eligible for endovascular therapy, prehospital triage to a more distant comprehensive stroke centre (CSC) compared with a closer primary stroke centre (PSC) was associated with significantly shorter time to thrombectomy, better...
In a recently published systematic review and meta-analysis, the use of protocol-based general anaesthesia (GA) in acute ischaemic stroke patients undergoing thrombectomy was significantly associated with less disability at three-months, compared with procedural sedation. However, the authors, Silvia Schönenberger...
Jacques Moret (Paris, France) talks to BLearning Neuro about his presentation titled ‘Past, present and future of neuroradiology’ at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy; 4–6 September 2019; Nice, France).
Moret says that the discipline of neuroradiology was, in the...
Guerbet announces that it has signed an exclusive agreement with icometrix for the distribution in France, Italy and Brazil of icobrain, their Saas (Software as a Service) artificial intelligence (AI)-based medical imaging solution.
Icobrain is designed to help radiologists and...
Insera Therapeutics has announced that the first two ischaemic stroke patients have been treated with its flagship cyclic aspiration system, CLEAR. The company received CE mark approval for its flagship product, the CLEAR Aspiration System, in March 2019.
“We used...
Medtronic announces it has filed a premarket approval (PMA) supplement with the US Food and Drug Administration (FDA) for approval of its InterStim Micro neurostimulator and also its InterStim SureScan MRI leads. InterStim Micro is a rechargeable, implantable sacral...
Axonics announced the presentation of detailed one-year results from its ARTISAN rechargeable sacral neuromodulation (r-SMN) pivotal study at a plenary session at the joint scientific meeting of the American Urogynecologic Society (AUGS) and the International Urogynecological Association (IUGA). The...
Abbott has received FDA approval for its Proclaim XR recharge-free neurostimulation system for people with chronic pain. A press release reports that the Proclaim XR platform offers a low dose of Abbott’s proprietary BurstDR stimulation waveform, which has been...
Axonics has announced that it has entered into an exclusive agreement to supply the Axonics rechargeable sacral neuromodulation (r-SNM) system to Adult Pediatric Urology and Urogynecology (“ADPU”), headquartered in Omaha, Nebraska.
ADPU is a pioneering institution in the SNM treatment...
LivaNova has announced the first patient enrolled in their RECOVER trial, a prospective, multi-centre, randomised controlled blinded trial demonstrating the safety and effectiveness of Vagus nerve stimulation (VNS) therapy as an adjunctive therapy versus a no stimulation control in...
Laurent Pierot (Reims, France) talks to BLearning Neuro at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy; 4–6 September; Nice, France) about the HPC (Hydrophilic Polymer Coating) surface modification technology (phenox) which he says is “very innovative” and could, in the future,...
Every two seconds, someone on earth will have a stroke. Every 10 seconds, stroke claims a life. With little respect for age, as a third of strokes occur in people below the age of 65, their aftermath can leave...
Highlights:
NeuroNews exclusively interviews Sheila Martins (Porto Alegre, Brazil) who provides an update on the RESILIENT team’s plan of action following the trial’s positive results, and details how the trial is set to shape stroke systems of care in developing...
Highlights:
NeuroNews exclusively interviews Sheila Martins (Porto Alegre, Brazil) who provides an update on the RESILIENT team's plan of action following the trial's positive results, and details how the trial is set to shape stroke systems of care in...
The Brainstorms Scientific are launching the Brainstorms Festival, a two-day business and human-orientated technology event set to take place in Vienna, Austria, on 27–28 September. The festival features several keynote talks and panel discussions addressing how understanding the human...
Aidoc, provider of artificial intelligence (AI) solutions to radiologists, has announced the release of its complete AI package for the identification and triage of stroke in CT scans. The CE-marked solution flags and prioritises vascular occlusions which result in...
Phenox announces that it has signed an exclusive distribution agreement with Wallaby Medical for commercialisation of the Avenir Detachable Coil System in the US and EU markets. The intended use for the Avenir Coil System is for endovascular embolisation...
In honour of September Pain Awareness Month, Stimwave has announced the launch of a new randomised clinical trial treating painful peripheral neuropathy, known as the “SMOOTH” study.
Painful peripheral neuropathy resulting from damage to the peripheral nervous system affects an...
Urs Fischer talks to BLearning Neuro at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy; 4–6 September 2019; Nice, France) about some of the differences regarding symptoms and treatment of stroke in children and adults.
Fischer says that stroke in children is...
David Putrino has helped innovate a variety of “provocative” technologies within the field of neuromodulation, such as those with the potential to achieve modulatory effects without directly stimulating the brain. Here, he discusses his ongoing research, and details some...
Saluda Medical has announced the award of CE mark and the European commercial launch of the Evoke ECAP-controlled, closed-loop spinal cord stimulation (SCS) system for the treatment of chronic pain. The world's first commercial implants were completed in The...
Patrick Brouwer speaks to NeuroNews about what he considers to be the most important developments in the neurointerventional field to date. As president of the European Society for Minimally Invasive Neurological Therapy (ESMINT), Brouwer touches on the society’s long-term...
Overlook Medical Center’s Atlantic Neuroscience Institute is set to participate in the MIND trial: a prospective, multicentre, randomised controlled trial (RCT) examining the Artemis Neuro Evacuation Device (Penumbra), a minimally invasive device used to remove intracerebral haemorrhage.
The study is designed...
At the recent European Society of Minimally Invasive Neurological Therapy (ESMINT; 4–6 September, Nice, France), the award for the best European publication in the Journal of NeuroInterventional Surgery (JNIS) 2019 was presented to Leif Sorensen and Lasse Speiser from...
Low pain scores after spinal cord stimulation (SCS) trials are predictive of successful SCS implants with high sensitivity, according to a recent study. The authors, Charles Odonkor (senior author), Vwaire Orhurhu and colleagues from Harvard Medical School, Boston, USA,...
According to Laura Stein and others from the Icahn School of Medicine at Mount Sinai, New York, USA, patients admitted with acute ischaemic stroke were twice as likely to receive endovascular therapy beyond February 2015—or what the authors have...
Axonics, a medical technology company that has developed and is commercialising a novel implantable rechargeable sacral neuromodulation (SNM) device for the treatment of urinary and bowel dysfunction, has announced the approval of the Axonics r-SNM system by the US Food...
Mika Niemelä (Helsinki, Finland) talks to BLearning Neuro about the ‘Decision making in unruptured aneurysms’ session he chaired at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy; 4–6 September; Nice, France).
Niemelä touches upon his own talk on the pathology of aneurysm walls...
New data from both the Evoke and Avalon studies reiterate the importance of objective neurophysiological measures and clinically meaningful outcomes. These studies evaluated the performance of the first closed-loop spinal cord stimulation (SCS) system (Evoke; Saluda Medical) that objectively...
Omar Ishrak, Medtronic’s chairman and CEO is to retire on 26 April 2020, following the end of the company’s current fiscal year. Also, the Medtronic Board of Directors has announced key leadership appointments as part of its multi-year, leadership...
Advertorial
In acute ischaemic stroke, fast and complete recanalisation of the occluded vessel is associated with improved outcomes. While morbidity and mortality in these patients can be high, achieving complete or near-complete reperfusion has been associated with significant clinical benefit...
Advertorial
While the treatment paradigm for stroke continues to evolve, even a quick review of the current literature will point to how complete reperfusion after a single thrombectomy pass is a predictor for favourable outcomes. During the Society of...
“The main reason to reappraise the concept of neuroprotection in acute stroke is the of effective thrombectomy, which de facto has led to the development of a reversible middle cerebral artery (MCA) occlusion model in humans. By reliably...
A study comparing three different stents used during stent-assisted coiling of cerebral aneurysms revealed that the type of stent used affects a patient’s immediate and long-term health outcomes, while a significant difference in the rate of complete occlusion among...
Twenty-four per cent of doctors are using social media daily to conduct medical research, and 85% of healthcare companies now implement social media marketing, says Georgios Matis. Although calling for patients and practitioners alike to “proceed with caution” when...
The 1st International Distal Thrombectomy Summit took place in North Italy, 14–15 June, 2019. According to a press release, the purpose of the meeting was to create a discussion about distal occlusions and explore questions that come to mind....
Abbott has announced that a new study, published in Lancet Neurology, found that elevated levels of a protein measured with the company's blood test under development could help detect mild traumatic brain injuries (TBIs), even when a CT scan...
Nevro has announced that it has completed patient enrolment in its prospective, multicentre randomised controlled trial (RCT) for the treatment of chronic pain for patients who suffer from painful diabetic neuropathy (PDN).
"This is an important step in the evaluation...
“Anatomical lead placement is a viable alternative to targeted lead placement for spinal cord stimulation,” concluded Jason E Pope, Evolve Restorative Center, Santa Rosa, USA, while presenting data from the DELIVERY trial, which evaluated anatomic versus targeted lead placement...
Three-year outcomes of the Surpass intracranial aneurysm embolisation system pivotal trial to treat large or giant wide neck aneurysm (SCENT trial) confirm the safety and efficacy of the device. Referring to the significant progression of aneurysm occlusion, Ricardo Hanel...
Fifty-six per cent of US neurointerventionalists meet criteria for burnout. This statistic, presented by Kyle Fargen from Wake Forest Baptist Health in Winston-Salem (USA) at the Society of NeuroInterventional Surgery’s (SNIS) annual meeting (22–25 July, Miami, USA), was based...
MRI Interventions said its ClearPoint Neuro navigation system has been used in 3,000 neurosurgical procedures.
The milestone procedure was completed at the University of California, San Francisco, USA, according to the vendor.
“This milestone is a testament to the value surgeons...
Siemens Healthineers will acquire all issued and outstanding shares of common stock of Corindus Vascular Robotics, Corindus announced this month.
The cash transaction is US$4.28 per share, resulting in an aggregate purchase price of US$1.1 billion by Siemens Medical Solutions,...
A low Alberta stroke program early CT score (ASPECTS) of 4–5 has been deemed the threshold for mechanical thrombectomy, with no benefit elicited in those with ASPECTS 0-3. Moreover, the clinical outcome in low ASPECTS may be age dependent,...
A study evaluating different waveforms within a wireless spinal cord stimulator (SCS) device (Stimwave) has found high response rates for patients receiving both high and low frequency waveforms. These data from the SURF trial were presented by Robert Bolash...
Does the shift away from open surgical vascular procedures towards percutaneous interventions favour a rise in the use of robotics? Gavin Britz (Houston Methodist Hospital, Houston, USA) argues yes, telling delegates at the Society of NeuroInterventional Surgery’s (SNIS) annual...
Patrick Brouwer (Stockholm, Sweden) talks to BLearning at SNIS 2019 (22–25 July, Miami, USA) about some of the issues associated with early flow diverters and why there is “not a lot of understanding going on with flow diverters at the moment”.
The “biggest...
According to a BIBA MedTech survey, 52% of neurointerventionalists believe that they are the only specialists who have the relevant skills and experience to perform interventional stroke procedures (e.g. mechanical thrombectomy). However, of those who believe other specialities—with the appropriate...
A progressive emergency medical services (EMS)–driven partnership in South Florida (USA) has expedited access to lifesaving care for stroke patients. This groundbreaking effort to optimise the likelihood of recovery from a “brain attack” was showcased this week at the...
Perfuze, a medical device company developing next-generation catheter-based technology to treat acute ischemic stroke, has been granted breakthrough device designation by the US Food and Drug Administration (FDA) for its Millipede Clot Ingestion System (CIS) technology.
Millipede CIS comprises a...
Donald Frei (Denver, USA) talks to BLearning at SNIS 2019 (22–25 July, Miami, USA) to discuss recent data from the cohort of thrombectomy trials which has shown a “benefit” for stroke patients with lower ASPECTS (Alberta Stroke Program Early CT Scores). “What...
A survey investigating the factors associated with the decision-making for endovascular thrombectomy (EVT) procedures found that such decisions differed significantly between regions and specialities depending on their local resources, but not under assumed ideal conditions. At the Society of...
Non-internal carotid artery (non-ICA) site of occlusion, the use of a balloon-guided catheter, and better collateral grade were all independent predictors of the first-pass effect, concluded Ashutosh Jadhav from the University of Pittsburgh, Pittsburgh, USA, at the recent Society...
In the largest cohort study to date, new research from Jefferson (Philadelphia University and Thomas Jefferson University, Philadelphia, USA) demonstrates that transradial surgery, done via the wrist, is safe and effective for a broad range of neuroendovascular procedures, and...
Important methodological aspects of clinical trials of spinal cord stimulation (SCS) using a placebo are consistently being poorly reported, says a recent systematic review. Within the context of the fast-evolving field that is neuromodulation, the authors, Rui V Duarte...
Matthew Gounis (Worcester, USA) talks to BLearning at SNIS 2019 (22–25 July, Miami, USA) about the current state of ischaemic stroke therapy. Gounis notes that seven randomised controlled trials conducted in the last five years have shown that mechanical thrombectomy...
Andrew Molyneux (Oxford, UK) spoke to BLearning following his luminary lecture at SNIS 2019 (22–25 July, Miami, USA) titled, "Reflections on 30 years of cerebral aneurysm treatment: The impact of the ISAT trial."
Molyneux explained that the International Subarachnoid Aneurysm Trial (ISAT) which...
Marc Ribo (Barcelona, Spain) talks to BLearning at SNIS 2019 (22—25 July, Miami, USA) about the benefits of optimising time to thrombectomy by cutting out imaging and going straight to the angiosuite.
Ribo says that in the acute phase of stroke...
Stimwave has announced completion of patient enrolment and preliminary results of the first-of-its-kind Tsunami study. The Tsunami placebo-controlled double-blind randomised clinical trial (RCT) is the first ever placebo-controlled double-blind RCT in the chronic pain relief neuromodulation industry. This study...
Medtronic and Viz.ai have partnered to accelerate the adoption of Viz.ai’s new technology, which helps synchronise stroke care and decrease time to treatment, potentially improving outcomes for patients.
Viz.ai’s technology uses artificial intelligence (AI) to identify suspected large vessel occlusion...
From tPA augmentation and stroke systems of care through to the rise of neuroprotective investigations, Santiago Ortega-Gutiérrez, University of Iowa (Iowa City, USA) kick started the Society of NeuroInterventional Surgery (SNIS) annual meeting (22–25 July, Miami, USA) by outlining...
Penumbra announces commercial availability of the Penumbra JET 7 reperfusion catheter with XTRA FLEX technology, at the Society of NeuroInterventional Surgery (SNIS) 16th Annual Meeting in Miami, USA.
Penumbra JET 7 with XTRA FLEX technology is used with the Penumbra...