 MagVenture has introduced the MagVenture Flow Arm — a patent-pending, clinical positioning system to optimise the delivery of MagVenture’s FDA cleared protocols for non-invasive brain stimulation technology within the treatment of major depressive disorder and as an adjunct therapy for obsessive compulsive disorder (OCD).
MagVenture has introduced the MagVenture Flow Arm — a patent-pending, clinical positioning system to optimise the delivery of MagVenture’s FDA cleared protocols for non-invasive brain stimulation technology within the treatment of major depressive disorder and as an adjunct therapy for obsessive compulsive disorder (OCD).
“Responsiveness to customer needs is a core part of MagVenture DNA,” says MagVenture SVP of Sales, Kerry Rome. “MagVenture is continuously striving to improve solutions to optimise the clinical practice of transcranial magnetic stimulation (TMS). In recent years, the clinical market for TMS has expanded at such a rapid pace that manufacturers are being challenged to keep pace with the technological requirements emerging with the exponential expansion in the demand for utilisation. We have seen MagVenture drive an FDA clearance for the ultra-fast ‘Theta Burst’ TMS which has reduced treatment times for patients from 37 to just three minutes. Now, with the introduction of a remarkedly smooth solution to help TMS operators quickly and easily prepare patients for treatment, MagVenture is further improving the efficiency and experience of TMS for patients and operators alike,” states Rome.
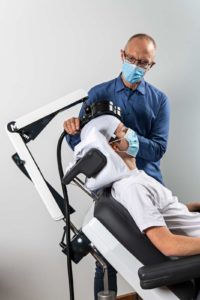
According to a company press release, the latest improvement to the MagVenture TMS Therapy system deemed the ‘MagVenture Flow Arm’, concerns the applicator ‘coil’. The coil is essentially two very heavy spools of copper wire that produce and focus the magnetic fields for the non-invasive brain stimulation technique. MagVenture state that the typically large and unwieldy coils, by necessity, have to be accurately positioned and held immobile over the brain target(s) during the TMS procedures.
The company reports that over the past year, MagVenture product engineers and application experts, in close collaboration with MagVenture customers in the USA, Europe, Australia, and Asia, perfected a solution that vastly improves ease of use for MagVenture TMS operators to now, almost effortlessly, move and securely position TMS coils for non-invasive brain stimulation (TMS) procedures.
Rome adds: “Throughout the research and development and testing phases for the Flow Arm project, the MagVenture organisation here in the US has been waiting with much anticipation and, finally, we are super excited about the introduction of MagVenture’s patent-pending ‘Flow Arm’ for TMS coil positioning. It is a game-changer for TMS providers seeking optimal ease of use and efficiency in their clinical TMS services and, in context with the current COVID-19 pandemic, the improvements in the MagVenture reduced treatment/operator-patient interaction time is going to prove to be a big positive.”