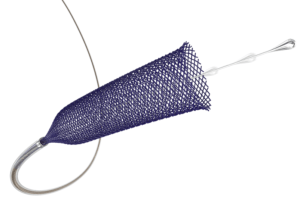
phenox has announced the European launch of the new p64 MW Flow modulation device, intended for the treatment of intracranial aneurysms. The device received CE mark in January 2020 and is available with the proprietary HPC (hydrophilic polymer coating) surface modification.
The p64 MW Flow modulation device is a next-generation stent-like device, implanted across the neck of a cerebral aneurysm, and is intended to divert blood flow away from the aneurysm. Over the course of several months, the diseased vessel is then reconstructed, permanently occluding the aneurysm from blood flow.
In addition to the novel HPC coating, the new p64 MW device includes new design features which improve deliverability, including a moveable wire and drawn filled tube construction for complete visibility under fluoroscopy. According to the company, the p64 MW is also 0.021” microcatheter compatible, making it the lowest profile 64-wire flow diverter available today.
phenox state that all p64 MW implants are available as a bare version as well as an HPC-coated version that provides additional safety in patients by mimicking the intact vessel wall. “HPC significantly reduces the thrombogenicity of our devices, by imitating the natural vessel wall without altering in vivo tissue response,” says Ing Hermann Monstadt, managing partner and founder of phenox. “With the recent introduction of our p64 MW HPC as well as the p48 MW HPC and pCONUS HPC bifurcation aneurysm implant we achieved to implement this technology on a full range of neurovascular implants for long-term use.”
The company’s release also speculates that surface modification of stents with HPC may also enable mono-antiplatelet therapy (MAPT) in patients for whom DAPT (double-antiplatelet therapy) is unsuitable.
phenox add that the development of HPC and its implementation on the p64 MW Flow Diverter system is a significant step toward improved patient care in vascular intervention in the up to 30% of patients in whom DAPT is ineffective or contraindicated. Although DAPT remains the standard of care for vascular implant patients, the reduced thrombogenicity with HPC enables device implantation under MAPT if there is no reasonable alternative therapy. This is justified in exceptional cases under guidance, and in conditions of intensive monitoring and response testing.













