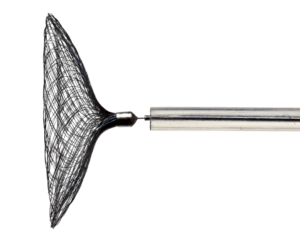
Cerus Endovascular has announced that the US. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application to conduct a US trial for the Contour Neurovascular System™, indicated for the treatment of intracranial aneurysms.
The IDE approval follows the receipt of Breakthrough Device Designation from the FDA in February 2021.
Stephen Griffin, president of Cerus Endovascular, said: “We are eager to move ahead with this important trial and anticipate patient enrollment beginning within the next three months
“The IDE study protocol closely aligns with protocols of other intra-saccular aneurysm repair devices that have received FDA approval. Given the real-world patient outcomes we have experienced in Europe, where the Contour Neurovascular System™ has had CE mark approval since March 2020, we are hopeful that we will see similar, strong results from this trial.”
The study is designed to develop a robust data set to support the safety and efficacy of the Contour Neurovascular System™ for the endovascular embolisation of wide-necked, bifurcated, saccular intracranial aneurysms. Study results will be submitted in a Premarket Approval (PMA) application to the FDA.
Sam Milstein, Cerus’ chairman, added: “International demand for the system has been strong. Since obtaining CE Mark last year, we have completed cases in 121 new partner institutions within 12 countries across Europe and Asia.
“During this time, we have accumulated substantial additional clinical data as well as 12-month post-operative follow-up, all of which indicates that the Contour Neurovascular System™ is well positioned to meet the requisite endpoints of the US study.”













