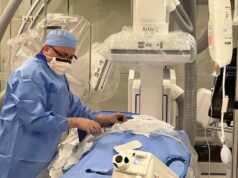
The US Food and Drug Administration (FDA) has cleared the SOMATOM On.site, a mobile head computed tomography (CT) scanner from Siemens Healthineers that enables a critically ill patient to receive CT head imaging in the intensive care unit (ICU) while remaining in bed.
The US Food and Drug Administration (FDA) has cleared the SOMATOM On.site, a mobile head computed tomography (CT) scanner from Siemens Healthineers that enables a critically ill patient to receive CT head imaging in the intensive care unit (ICU) while remaining in bed.
Performing a CT head examination at the patient’s bedside can eliminate costly transports to the radiology department, which involve high staffing requirements and potential patient risk.
“The SOMATOM On.site transforms the delivery of care for critically ill patients who require a CT head scan,” says Douglas Ryan, vice president of Computed Tomography at Siemens Healthineers North America. “The system delivers reliable and consistent image quality demanded by healthcare professionals in the ICU, neurology, and radiology departments. Additionally, bedside imaging helps to reduce patient transports, thereby reducing the risk of infection while improving workforce efficiency.”
A press release from the company states that the SOMATOM On.site offers easy scan setup, fast workflow, and integrated patient support accessories, for convenient and consistent patient positioning in the scanner. An intelligent user interface concept, myExam Companion helps the radiologic technologist navigate the examination for consistent results.
An integrated drive camera enables real-time viewing on the built-in Touch UI display for easy manoeuvring by the technologist. The scanner’s unique telescopic gantry design allows the radiation source to move away from the patient during scanning, while the base and the front cover of the gantry remain stationary. Moreover, the company states that the SOMATOM On.site system design—with the telescopic, self-shielded gantry and attachable front and back radiation shields—reduces scatter radiation compared to CT scanners without this design and provides radiation protection for neighbouring patients and staff.