Tag: Neuromodulation
North American Neuromodulation Society announces 2025 award recipients ahead of annual...
The North American Neuromodulation Society (NANS) has announced its 2025 award winners ahead of the NANS annual meeting (30 January–1 February, Orlando, USA), recognising...
Carryover effect exists and has “remarkably high” variability in spinal cord...
An international, multicentre study has confirmed the existence of the carryover or ‘echo’ effect—an interval before patients perceive the return of pain after their...
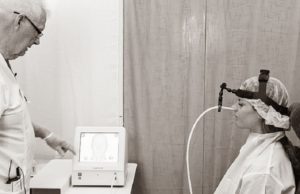
Study reinforces ‘widely held but underexplored’ view that ECT is safe...
Researchers have found that electroconvulsive therapy (ECT)—whereby an electric current is passed through the brain of a patient under general anaesthesia to induce a...
Mainstay announces publication of five-year follow-up data from ReActiv8-B trial
Mainstay Medical has announced the publication of the five-year follow-up from its ReActiv8-B randomised, sham-controlled, double-blinded trial in Neuromodulation: Technology at the Neural Interface.
There...
Medical necessity “should drive all remote monitoring decisions” in SCS, expert...
The principle of medical necessity “should drive all remote monitoring decisions” relating to spinal cord stimulation (SCS) technologies, according to recommendations recently published in...
SynerFuse completes enrolment in proof-of-concept study
SynerFuse has announced that it has completed enrolment in its proof-of-concept study to evaluate the safety and tolerability of the Electric Transforaminal Lumbar Interbody...
Biotronik announces new data supporting effectiveness of RESONANCE multiphase stimulation paradigm
Biotronik Neuro has announced that results from the BENEFIT-02 trial—the “first of its kind” to clinically evaluate a multiphase stimulation paradigm—support the effectiveness of...
Medtronic names Paolo Di Vincenzo president of its neuromodulation business
Medtronic has shared that Paolo Di Vincenzo will join the company as president of its neuromodulation business, effective 30 October 2023. In this role,...
Intrathecal drug delivery effective for managing cancer-related pain, as per existing...
Two separate systematic reviews and meta-analyses—both published recently in Neuromodulation: Technology at the Neural Interface—have concluded that intrathecal drug delivery (IDD) systems are effective...
Novel ‘infraslow pink noise’ stimulation found to be safe and feasible...
A novel brain stimulation technique called high-definition transcranial infraslow pink noise stimulation (HD-tIPNS) was recently evaluated in a placebo-controlled pilot study, and found to...
RECOVER trial will provide “thorough understanding” of VNS response in depression...
A new randomised controlled trial (RCT)—the “largest ever” of its kind in the major depressive disorder (MDD) space—will attempt to provide a “thorough understanding”...
Office-based removal of sacral neuromodulation devices deemed safe and well-tolerated
As per a recently published retrospective chart review, the removal of sacral neuromodulation (SNM) devices in the office setting, under local anaesthesia, is a...
Evidence indicates effectiveness of brain stimulation in upper-limb stroke rehabilitation
Moderate- to high-quality evidence suggests that specific neuromodulation modalities can provide effective treatments in patients undergoing stroke rehabilitation. As per a systematic review and...
European Society for Brain Stimulation “strongly opposes” reclassification of non-invasive devices
The European Society for Brain Stimulation (ESBS) has said it “strongly opposes” the European Union’s (EU) recent decision to reclassify non-invasive brain stimulation (NIBS)...
High-frequency spinal cord stimulation may be associated with “significant decrease” in...
High-frequency spinal cord stimulation (HF-SCS) for treating chronic refractory low back pain (CRLBP) may be associated with a significant decrease in total healthcare costs,...
Randomised trial finds active tDCS to be effective treatment in women...
A randomised, triple-blinded clinical trial involving 35 women has found that active transcranial direct current stimulation (tDCS)—delivered at an intensity of 2mA and across...
Nalu Medical announces publication of two spinal cord stimulation clinical studies
Nalu Medical—a company working on miniaturised neurostimulation implants for the management of chronic intractable pain via both spinal cord stimulation (SCS) and peripheral nerve...
Intermittent stimulation of the DRG produces comparable chronic pain results to...
Intermittent dorsal root ganglion stimulation (DRG-S) has been shown to produce comparable results to continuous stimulation across a two-week period, as reported in Neuromodulation:...
Progress update on Women in Neuromodulation: Achievements and obstacles
In light of recent progress made in alleviating gender disparities within the neuromodulation field, Magdalena Anitescu (Chicago, USA), Anne Fennimore (Boca Raton, USA), Kiran...
Phase one winners announced in US$9.8 million Neuromod Prize competition
The National Institutes of Health (NIH) Common Fund has named eight winners of the first phase of the Neuromod Prize—a US$9.8 million competition to accelerate...
Current literature suggests promise held by electrical stimulation therapies for cerebral...
Electrical stimulation technologies, and in particular spinal cord stimulation (SCS) and sphenopalatine ganglion stimulation, offer a promising adjunct to existing therapies for cerebral vasospasm...
Neuromodulation offers hope for patients with long-COVID
With increasing focus being placed on the long-term impact COVID-19 can have, and the demand for effective treatments also growing concurrently, Marom Bikson (New...
Neuromodulation for post-stroke recovery advancing on several fronts
As a principal investigator for the pivotal VNS-REHAB study, which supported the first US Food and Drug Administration (FDA) premarket approval of a vagus...
Millimetric implant offers promising alternative to opioids or surgery in pain...
Using a one-millimetre-sized wireless implant to stimulate peripheral nerves from within blood vessels has the potential to treat neuropathic pain that is resistant to...
Multiple daily doses of iTBS provide no additional benefit in treatment-resistant...
In patients with treatment-resistant depression (TRD), multiple sessions of intermittent theta-burst stimulation (iTBS) do not accelerate responses to the therapy, nor do they improve...
AI, cost reductions and more represent bright future for neuromodulation industry
On technological and commercial fronts alike, the neuromodulation space is one of the more rapidly evolving subsectors within the medical industry—and has undergone a...
Registration opens for 15th International Neuromodulation Society congress in Barcelona
At the International Neuromodulation Society (INS) 15th world congress (21–26 May 2022; Barcelona, Spain), researchers from over 40 countries will present plenary lectures and...
Single-patient study offers promise for individualised depression treatment with DBS
Despite its small sample size, a study in which a patient received closed-loop, deep brain stimulation (DBS) therapy has demonstrated positive clinical outcomes and...
Researchers pave way for adaptive DBS treatment after identifying OCD-associated brain...
In an effort to improve treatment for obsessive-compulsive disorder (OCD), a team of researchers has—for the first time, according to a Brown University (Providence,...
NANS 2022: New 18-month data show durability of high-frequency SCS for...
This year’s North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA) saw 18-month data from SENZA-PDN—a randomised controlled trial (RCT) evaluating the...
Salim Hayek
Salim Hayek is chief of the Division of Pain Medicine at University Hospitals of Cleveland and a professor in Case Western Reserve University’s Department...
Feinstein Institutes scientists develop “first-of-its-kind” wireless neuromodulation device for research
Engineers and researchers in the Institute of Bioelectronic Medicine at the Feinstein Institutes for Medical Research have developed a novel, fully implantable, wireless bidirectional vagus nerve stimulation (VNS)...
NIH launches first phase of US$9.8 million competition to accelerate development...
The National Institutes of Health (NIH) has launched the first phase of the Neuromod Prize—a US$9.8 million competition to accelerate the development of neuromodulation therapies....
Nevro announces clinical data reinforcing significant and durable benefits of 10kHz...
Nevro Corporation has announced the results from data presentations at the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA) supporting...
Novel neuromodulation technique could be “gamechanger” in treatment-resistant depression
Stanford accelerated intelligent neuromodulation therapy (SAINT)—a novel, high-dose intermittent theta-burst stimulation (iTBS)—was found to be safe and more effective than sham stimulation in a...
FDA grants Breakthrough Device designation to Wavegate for StimuLux system
Wavegate Corporation has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for its StimuLux optical reflectometry system for closed-loop...
Mainstay Medical announces positive efficacy and safety outcomes from ReActiv8-B trial
Mainstay Medical has announced the publication of two-year patient outcomes data from its pivotal ReActiv8-B clinical trial. The data, which are published in Neuromodulation:...
Boston Scientific to present new data on advances in chronic pain...
Boston Scientific has announced key data that will be featured at the 25th North American Neuromodulation Society (NANS) annual meeting (13–15 January 2022; Orlando, USA)....
Current gaps and barriers in patient access to spinal cord stimulation
Maricela Schnur (St Luke’s Hospital, Duluth, USA) highlights a handful of socioeconomic disparities, racial differences, and psychological and psychiatric factors, that currently present difficulties...
Mouse study suggests neuromodulation of certain nerve cells can help regenerate...
Johns Hopkins Medicine researchers say they have new evidence from mouse experiments that manipulating certain nerve cells, or the genes that control them, with...
FluxWear to debut wearable neuromodulation device at 2022 Consumer Electronics Show
FluxWear has announced that it will be demonstrating its first product at the Consumer Electronics Show (CES) early next year (5–8 January 2022, Las...
New protocol may reduce need for psychological assessments prior to neuromodulation...
Researchers at Toronto Western Hospital in Canada have proposed a protocol that may precede in-person assessments prior to patients receiving neuromodulation therapies. Their protocol...
BlueWind Medical completes patient enrolment in OASIS study
BlueWind Medical has announced the successful completion of patient enrolment in the OASIS pivotal clinical study that will assess the company’s innovative Renova iStim...
NeuroPace gains IDE approval to launch pivotal study of neuromodulation therapy...
NeuroPace has announced that it has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to initiate the NAUTILUS...
Research shows brain rhythm changes occur ‘within minutes’ of DBS surgery...
New research published in Translational Psychiatry presents evidence that brief intraoperative exposure to therapeutic deep brain stimulation (DBS) at the time of implantation surgery induces...
Nevro announces plans to appeal jury decision in spinal cord stimulation...
Nevro Corporation has provided an update on its position following a Delaware jury’s decision to award Boston Scientific US$20 million over patent infringements. The...
Rune Labs and Medtronic partner to improve understanding of neurostimulation with...
Rune Labs, a company using aggregated brain data to empower the development and delivery of precision medicines for neurological and psychiatric diseases, and global healthcare...
Magnus Medical announces Breakthrough Device designation for novel depression treatment and...
Magnus Medical has announced that the US Food and Drug Administration (FDA) has granted the company Breakthrough Device designation for its individualised, rapid-acting, non-invasive...
SPR Therapeutics announces expanded FDA clearance for SPRINT peripheral nerve stimulation...
SPR Therapeutics has obtained clearance from the US Food and Drug Administration (FDA) of a broader indication for the usage of its SPRINT peripheral...
Study indicates safety and efficacy of remote electrical neuromodulation in chronic...
Theranica has announced the publication of a new, peer-reviewed article in Pain Reports detailing a study assessing its novel, drug-free treatment option for chronic...
High-frequency SCS takes the stage to relieve chronic pain from diabetic...
Following the recent approval of the Senza system (Nevro) by the US Food and Drug Administration (FDA), Erika A Petersen (Little Rock, USA) discusses the...
CRISP study indicates clinical benefit of non-paraesthesia-based approaches in burst SCS...
The CRISP (Comparison of paraesthesia mapping to anatomic midline-based burst programming strategies) study, published in Neuromodulation: Technology at the Neural Interface, has found that...
Boston Scientific launches FAST—a new therapy for spinal cord stimulation
Boston Scientific has announced the European launch of FAST—a new, fast-acting sub-perception therapy that the company says is clinically proven to demonstrate significant and...
Neuromod Devices appoints Diarmuid Flavin as COO to boost expansion of...
Neuromod Devices has announced the appointment of Diarmuid Flavin as chief operating officer (COO). Flavin brings more than 25 years of global leadership experience...
MicroTransponder gains FDA approval for first-of-its-kind VNS system to improve stroke...
MicroTransponder has announced US Food and Drug Administration (FDA) premarket approval of the Vivistim paired vagus nerve stimulation (VNS) system, which—according to a company...
Presidio Medical publishes clinical and preclinical research on ULF neuromodulation therapy
Presidio Medical has announced that research involving its novel ultra-low frequency (ULF) neuromodulation therapy has been published in Science Translational Medicine. The publication, titled...
Temporary SCS could represent “new paradigm” in preventing atrial fibrillation
In a randomised pilot study, temporary spinal cord stimulation (SCS) has demonstrated efficacy in the suppression of postoperative atrial fibrillation (AF) following coronary artery...
Revolution in neurotherapy? Spotlight on focused ultrasound at 2021 EAN congress
While focused ultrasound is by no means a brand-new innovation in the field of neurotherapy—having first been deployed in the 1950s, if not earlier,...
Long-term effectiveness of STN-DBS for Parkinson’s confirmed with 15-year follow-up study
New findings published in Neurology, the medical journal of the American Academy of Neurology (AAN), have confirmed the long-term effectiveness of deep brain stimulation...
Nevro announces FDA approval of 10kHz high-frequency SCS therapy for painful...
Nevro has announced receipt of US Food and Drug Administration (FDA) approval for its Senza system for the treatment of chronic pain associated with...
How stimulation is changing the future of mental health treatment
Daniel Månsson (Flow Neuroscience, Malmö, Sweden) outlines the benefits held by transcranial direct-current stimulation (tDCS) in the treatment of depression—and how clinical evidence supporting...
Men and women respond equally well to spinal cord and DRG...
In a study intended to explore gender differences in patients with failed back surgery syndrome (FBSS) or chronic visceral pain, men and women have...
Mainstay Medical launches implantable restorative neurostimulation system in USA
Mainstay Medical has announced the limited US commercial launch of ReActiv8, its implantable restorative neurostimulation system to treat intractable chronic low back pain. The...
Benefits found in directional DBS compared to omnidirectional stimulation for Parkinson’s...
Directional deep brain stimulation (DBS) has demonstrated benefits over omnidirectional DBS for the treatment of Parkinson’s disease in the largest prospective study in this...
New algorithm could enable next-generation deep brain stimulation devices
By delivering small electrical pulses directly to the brain, deep brain stimulation (DBS) can ease tremors associated with Parkinson's disease or help relieve chronic...
Study shows “major energy savings” can be achieved in spinal cord...
The results of the HALO (High and low frequency options) study, which are published online in the scientific journal Neuromodulation: Technology at the Neural...
Chordate Medical receives CE mark to treat chronic migraine with neuromodulation...
Chordate Medical has received a CE marking that permits its Kinetic Oscillation Stimulation (KOS) technology to be marketed, sold and used for the treatment...
rTMS found to be “well-accepted” for therapeutic use in psychiatric populations
Psychiatric populations perceive the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) for mental health conditions to be beneficial, safe, and no more dangerous...
Medtronic gains FDA approval to trial implantable tibial neuromodulation device
Medtronic has announced approval from the US Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate its...
Greater outreach needed for women and minority pain patients
During a presentation at the North American Neuromodulation Society (NANS) virtual meeting (15–16 January) entitled “Outcomes and Access to Neuromodulation for Women and Underrepresented...
Accessing neuromodulation: Race and ethnicity “should not cloud treatment options” for...
Stephanie Vanterpool (Knoxville, USA) talks to NeuroNews about how outreach can be used to address the disparities between white and underrepresented minority patients when...
ShiraTronics receives FDA breakthrough device designation for chronic migraine therapy
ShiraTronics has announced that it has been granted a breakthrough device designation by the Center of Devices and Radiological Health (CDRH) of the US...
Vercise DBS receives breakthrough device designation from FDA
Functional Neuromodulation has announced that the Vercise deep brain stimulation (DBS) device, developed and manufactured by Boston Scientific, has received breakthrough device designation from...
Research shows better cognition with non-invasive vagal nerve stimulation
In a study published this week in Neuromodulation: Technology at the Neutral Interface, authors report that they have initial evidence that non-invasive vagal nerve...
Dorsal root ganglion stimulation evokes motor responses in patients with complete...
Bilateral L4 dorsal root ganglion (DRG) stimulation has been shown to evoke strong and reproducible motor responses in the upper leg in patients with...
New study shows significant positive impact of vagus nerve stimulation therapy...
Adding vagus nerve stimulation (VNS) therapy to treatment as usual has been demonstrated to improve outcomes in patients with treatment-resistant bipolar depression (TRBD). In...
FDA approves Abbott’s iOS-compatible app for the management of chronic pain...
Abbott announces it has received approval from the US Food and Drug Administration (FDA) for the use of its Patient Controller app on compatible...
Asleep surgery improves the therapeutic window for deep brain stimulation
Evidence in support of the motion that asleep surgery improves the therapeutic window for deep brain stimulation (DBS) when compared to awake surgery, has...
Soterix wins IDE for depression treatment trial
Soterix Medical has announced that it received US Food and Drug Administration (FDA) investigational device exemption (IDE) to launch a trial of its non-invasive transcranial...
RECOVER trial design evaluating vagus nerve stimulation for treatment-resistant depression published
LivaNova announces a publication in Contemporary Clinical Trials, which details the design for a prospective, multicentre, randomised controlled blinded trial demonstrating the safety and...
Mainstay Medical announces FDA approval of ReActiv8 neurostimulation system for chronic...
Mainstay Medical has announced that the US Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for ReActiv8, its implantable...
Decision-making in intrathecal drug delivery devices “life-threatening” amid COVID-19
Amid the COVID-19 pandemic, the need to prevent the spread of disease and manage critical hospital resources has never been more critical. In a...
Non-invasive vagus nerve stimulation may provide clinical benefit to patients with...
An article accepted into the journal Neuromodulation has concluded that, based on two case reports, non-invasive vagus nerve stimulation (nVNS) might provide clinical benefits...
Educational curriculum for spinal cord stimulation developed to fill “unmet need”
A spinal cord stimulation (SCS) training curriculum has been developed by a multidisciplinary taskforce of the North American Neuromodulation Society’s (NANS) education committee. It...
Cervical non-invasive vagus nerve stimulation effectively relieves pain in migraine sufferers
A recent meta-analysis demonstrated that cervical non-invasive vagus nerve stimulation (nVNS) is a safe and effective method for acute pain relief in migraine and...
Novel intermittent dosing burst spinal cord stimulation deemed safe and efficacious...
A novel intermittent dosing burst paradigm in spinal cord stimulation (SCS) has effectively relieved pain in patients with chronic intractable pain. Authors Timothy Deer,...
LivaNova collaborates with Verily to fuel research into vagus nerve stimulation...
LivaNova has announced a research collaboration with Verily, an Alphabet company, to capture measures of depression within its RECOVER clinical study, which is evaluating...
News from NANS: 10kHz safe and effective for treating painful diabetic...
A multicentre study across 18 centres finds 10kHz safe and effective for the treatment of painful diabetic neuropathy. Sensory improvements were observed in many...
Stratus Medical completes private placement financing and acquisition of the NimbusRF
Stratus Medical, a new company created to focus on advancing radiofrequency (RF) ablation treatment for chronic pain, has announced that it completed a private...
News from NANS: First long-term vagus nerve stimulation implant in mice
Data showing the effective use of a long-term vagus nerve implant in mice have been presented at the North American Neuromodulation Society’s (NANS) annual...
Centres of excellence for neuromodulation: A critical proposal
While recognising that excellence derives from experience, Robert M Levy suggests that the neuromodulation community can better protect the field’s “desire to ensure excellence...
Abbott receives expanded indication from FDA for directional DBS to treat...
Abbott has announced it received approval from the US Food and Drug Administration (FDA) for a new, expanded indication for the company's Infinity deep...
News from NANS: BurstDR stimulation reduces negative psychological effects associated with...
New quality of life and functional improvement data from both the BOLD and TRIUMP studies echo the benefits of the Proclaim XR neurostimulation system...
SPRINT peripheral nerve stimulation system demonstrates 12 months of low back...
SPR Therapeutics has announced publication of a prospective case series regarding its SPRINT peripheral nerve stimulation (PNS) system in the treatment of low back...
Medtronic receives CE mark for InterStim Micro Neurostimulator and InterStim SureScan...
Medtronic has announced it has received CE mark for its InterStim Micro neurostimulator and InterStim SureScan MRI leads—clearing the technologies for commercial sale and...
Medtronic receives CE mark approval for the Percept PC Neurostimulator deep...
Medtronic announces the CE mark for Percept PC neurostimulator. It is the only Deep Brain Stimulation (DBS) system to be launched in the European...
Axonics granted six US patents and allowed three additional patents related...
Axonics has announced that the United States Patent and Trademark Office has issued or allowed Axonics nine US utility patents in 2019, along with...
Axonics provides survey results from Physician Seminar Series showcasing the recently...
Axonics, a medical technology company that has developed and is commercialising novel implantable Sacral Neuromodulation (SNM) devices for the treatment of bladder and bowel...
First educational module for neuromodulation ensures field is “future-proof”
The first educational module for neuromodulation, a masters-level e-learning course aimed at nurses and allied healthcare professionals working within the field, received its first...
New data demonstrates “unprecedented” efficacy outcomes with the NeuroPace RNS system
NeuroPace has announced that new data from a multicentre, real-world clinical study of the RNS system has demonstrated outcomes never before seen with other...
Pooled analysis finds dorsal root ganglion stimulation “safe and effective” for...
Dorsal root ganglion (DRG) stimulation is a safe and effective therapy for multiple chronic pain disorders, concludes a pooled analysis published in Neuromodulation. The...
Axonics announces US Food & Drug Administration approval for its sacral...
Axonics, a medical technology company that has developed and is commercialising novel implantable rechargeable sacral neuromodulation (SNM) devices for the treatment of bladder and...
Over-the-skin electrical stimulation helps provide movement in quadriplegics
Researchers at The Feinstein Institutes for Medical Research used new closed-loop neurostimulation methods and textile-based electrodes to facilitate individual finger movement and grasp force...
Nevro announce launch of a new medical device for the treatment...
Nevro today announced it has received approval from the US Food and Drug Administration (FDA) for the Senza Omnia spinal cord stimulation (SCS) system....
Axonics announces first commercial US patient implanted with its sacral neuromodulation...
Axonics has announced the first US-based implantation of the Axonics r-SNM system subsequent to its clearance by the US Food & Drug Administration (FDA)...
GTX Medical and NeuroRecovery Technologies announce merger
GTX Medical (formerly G-Therapeutics) and NeuroRecovery Technologies (NRT) have announced a merger, creating a global company for the development of neuromodulation therapies to aid...
Boston Scientific announces CE mark approval of the VERCISE Neural Navigator...
Boston Scientific has announced the CE mark approval of the Vercise Neural Navigator 3 directional deep brain stimulation (DBS) programming software—the first system that...
Medtronic announces FDA submission for InterStim Micro neurostimulator and SureScanTM MRI...
Medtronic announces it has filed a premarket approval (PMA) supplement with the US Food and Drug Administration (FDA) for approval of its InterStim Micro...
Axonics provides full one-year results from ARTISAN-SNM pivotal study
Axonics announced the presentation of detailed one-year results from its ARTISAN rechargeable sacral neuromodulation (r-SMN) pivotal study at a plenary session at the joint...
FDA approves low-dose spinal cord stimulator for chronic pain
Abbott has received FDA approval for its Proclaim XR recharge-free neurostimulation system for people with chronic pain. A press release reports that the Proclaim...
Axonics announces agreement to supply sacral neuromodulation systems to leading urology...
Axonics has announced that it has entered into an exclusive agreement to supply the Axonics rechargeable sacral neuromodulation (r-SNM) system to Adult Pediatric Urology...
The Brainstorms Scientific set to host Europe’s first transdisciplinary neuroscience festival
The Brainstorms Scientific are launching the Brainstorms Festival, a two-day business and human-orientated technology event set to take place in Vienna, Austria, on 27–28...
Stimwave launches post-market peripheral neuropathy pain relief randomised controlled trial
In honour of September Pain Awareness Month, Stimwave has announced the launch of a new randomised clinical trial treating painful peripheral neuropathy, known as...
Turning neuromodulation on its head: Fresh perspectives on interacting with the...
David Putrino has helped innovate a variety of “provocative” technologies within the field of neuromodulation, such as those with the potential to achieve modulatory...
Low pain scores following spinal cord stimulation trials found predictive of...
Low pain scores after spinal cord stimulation (SCS) trials are predictive of successful SCS implants with high sensitivity, according to a recent study. The...
Axonics announces US FDA approval for its sacral neuromodulation system
Axonics, a medical technology company that has developed and is commercialising a novel implantable rechargeable sacral neuromodulation (SNM) device for the treatment of urinary and...
Disruptive innovation in neuromodulation: What can we learn from #SocialMedia disrupting...
Twenty-four per cent of doctors are using social media daily to conduct medical research, and 85% of healthcare companies now implement social media marketing,...
Similar success rates for anatomic and targeted lead placement for spinal...
“Anatomical lead placement is a viable alternative to targeted lead placement for spinal cord stimulation,” concluded Jason E Pope, Evolve Restorative Center, Santa Rosa,...
The first wireless spinal cord stimulator system elicits a high response...
A study evaluating different waveforms within a wireless spinal cord stimulator (SCS) device (Stimwave) has found high response rates for patients receiving both high...
Placebo-controlled RCTs of spinal cord stimulation are on the rise, but...
Important methodological aspects of clinical trials of spinal cord stimulation (SCS) using a placebo are consistently being poorly reported, says a recent systematic review....
Stimwave announces completion of enrolment for the Tsunami placebo-controlled double-blind randomised...
Stimwave has announced completion of patient enrolment and preliminary results of the first-of-its-kind Tsunami study. The Tsunami placebo-controlled double-blind randomised clinical trial (RCT) is...
The “neurosurgical robot” facilitates further research in deep brain stimulation
The application of robot-assisted lead implantation in nonhuman primate neuromodulation research has been deemed feasible, accurate, safe and efficient in a recent study published...
NIH study to utilise NeuroPace RNS system for research on the...
NeuroPace has announced that the company’s RNS system, a closed-loop neuromodulation technology approved to treat adults with medically refractory focal onset epilepsy, will be...
The first non-invasive, adaptive neuromodulation digital treatment for migraine receives CE...
Neurolief, a developer of digital therapeutics brain neuromodulation technology, has announced that it has received the CE mark for its Relivion non-invasive, adaptive digital treatment...
Latest FDA caution for the use of off-label drugs in intrathecal...
While the US Food and Drug Administration (FDA) has called for caution regarding the use of off-label medications administered intrathecally, pain management experts have...
New published data affirms BrainsWay’s deep TMS system for the treatment...
BrainsWay has announced new data from an article published in the lastest issue of the American Journal of Psychiatry demonstrating that its deep TMS (dTMS) system...
Cybersecurity: Providers and patients beware
Within the context of evolving technologies that continue to expand healthcare connectivity, Padma Gulur warns of the “immense vulnerability of current systems”. Arguing that...
Medication-free treatment for depression launches in the UK
Flow launches a medication-free treatment for depression comprising a brain stimulation headset and therapy app. In Europe, Flow is classified as a Class IIa...
Burst stimulation of the spinal cord leads to greater pain modulation...
Data from a substudy of SUNBURST suggest that burst stimulation of the spinal cord regulates the medial pain pathway to a greater extent than...
“A failure in SCS is not a failure of the therapy...
Defining true success as “being able to decrease or even eliminate a patient’s dependency on oral opioids”, Corey W Hunter, founder of Ainsworth Institute...
Review shows inaccuracies of deep brain stimulation data for minimally conscious...
Results from a systematic review reveal that evidence for the use of deep brain stimulation (DBS) of various targets to promote recovery in patients...
International Neuromodulation Society announces 14th World Congress
The International Neuromodulation Society (INS) will hold its 14th World Congress from May 25–30, 2019 in Sydney, Australia. The multidisciplinary meeting highlights top international...
Study of SPR Therapeutics’ peripheral nerve stimulation system demonstrates sustained relief...
SPR Therapeutics have announced the study results of its SPRINT peripheral nerve stimulation (PNS) system in the treatment of post-amputation pain.
The study demonstrated that...
Welkin Health announces partnership to implement a programme to support commercialisation...
Welkin Health, a privately-held patient relationship management software company has announced that it is partnering with Axonics, a company focused on the design, development,...
New data show the therapeutic benefits of directional leads for use...
The PROGRESS study has achieved its primary endpoint and demonstrated superiority of the therapeutic window using Abbott directional stimulation compared to omnidirectional stimulation. Results...
Physicians’ Education Resource to host first annual International Congress on the...
Physicians’ Education Resource (PER), a worldwide leading resource for continuing medical education (CME), will host the first annual International Congress on the Future of...
Evoke: The first RCT to measure spinal cord activations in response...
The protocol design for a multicentre, prospective, randomised, double-blind study comparing ECAP- (evoked compound action potential) controlled closed-looped with open-loop, fixed output spinal cord...
Positive results for the treatment of medication-resistant epilepsy using Neuroelectrics’ Starstim
At the recent American Epilepsy Society Annual Meeting in New Orleans, USA, Neuroelectrics presented positive results from its clinical trial treating patients with drug...
Axonics granted full-body MR-conditional CE mark for its sacral neuromodulation system
Axonics has announced that it has received CE mark approval for 1.5T and 3T full-body magnetic resonance imaging conditional labelling for the Axonics r-SNM...
Axonics announce positive clinical data from its ARTISAN sacral neuromodulation pivotal...
Axonics has disclosed positive top-line results from the ARTISAN-SNM pivotal study, designed to gain marketing approval from the US Food and Drug Administration (FDA)...
The future of neurostimulation: Smart neuromodulation
Better understanding of the brain alongside the integration of artificial intelligence may represent the future of neuromodulation. From machine learning and novel technology to...
Majority of radiculopathy patients prefer burst to conventional tonic stimulation
BurstDR stimulation confers a greater reduction in leg and back pain intensity on patients with failed back surgery syndrome that tonic stimulation does, a...
The top 10 takeaways from “The Neuromodulation Appropriateness Consensus Committee on...
In 2018, the International Neuromodulation Society (INS) project on Consensus Conferences published “The Neuromodulation Appropriateness Consensus Committee on Best Practices for Dorsal Root Ganglion...
Companies partner for a non-invasive neuromodulation device to treat spasticity
WeHealth by Servier, the e-health department of Servier Group, and PathMaker Neurosystems, have announced the closing of a partnership agreement to develop and commercialise...
Nuvectra receives full-body MR-conditional CE Mark approval for Algovita
Nuvectra has announced that it has received full-body MR-conditional approval for the Company’s Algovita spinal cord stimulation (SCS) system from its European Notified Body,...
Good news for gamblers: Transcranial direct current stimulation found to enhance...
Transcranial direct current stimulation (tDCS) offers a novel intervention for individuals with gambling disorder, concluded a recent study that found that its use enhanced...
Stimwave receives FDA clearance for first Wireless SCS System with iPhone-iWatch...
Stimwave Technologies has announced that it has received US Food and Drug Administration (FDA) clearance for the WaveCrest Mobile iOS platform patient controllers for...
SPR Therapeutics awarded US$10M to develop its SPRINT peripheral nerve stimulation...
SPR Therapeutics has been awarded two new grants and one new contract from the US Department of Defense totalling US$10M to further develop its...
SPR Therapeutics announces first use of SPRINT extensa dual-lead peripheral nerve...
SPR Therapeutics, a developer of neurostimulation technology for pain management, has announced that Christopher Gilmore of Carolinas Pain Institute was the first to use...
PathMaker Neurosystems launches European clinical trial of MyoRegulator
PathMaker Neurosystems has announced that it has initiated and successfully enrolled the first patient in its European clinical trial to evaluate MyoRegulator for the non-invasive treatment...
Intellis is now licensed in Canada
Medtronic has announced in a press release that it has received a licence from Health Canada for its Intellis platform, which includes the world's...
Algovita SCS System implantations reach 2,000 in the USA
Nuvectra Corporation, a neurostimulation medical device company, has announced that the Company’s Algovita Spinal Cord Stimulation (SCS) system has been implanted in over 2,000...
Study lays foundation for treating drug, alcohol addicts with noninvasive brain...
In a study investigating the use of transcranial magnetic stimulation (TMS) for drug addiction, researchers at Medical University of South Carolina are the first...
Axonics secures US$40M financing to commercialise neuromodulation device
Axonics has secured US$40 million to support commercialisation of its neuromodulatory treatment of urinary and bowel dysfunction. The financing comes as Axonics closes in on the...
Soin Neuroscience commences a clinical case series with Nuvectra’s Algovita spinal...
Soin Neuroscience, a biotech company based in Dayton, Ohio, USA, has received IRB (institutional review board) approval to commence a clinical case series to study...
Neurostimulation can be part of the solution to the opioid crisis
The president of the United States of America has recently declared the opioid crisis in that country a “public health emergency”. In light of...
“Shuffle” stimulation improves pain scores in pilot study
A pilot study published in Neuromodulation: Technology at the Neural Interface suggests that high frequency stimulation (HFS), an alternative SCS waveform could also be...
Innovation. Outcomes. Focus on Function.
Medtronic has a long-standing commitment to delivering innovative pain stimulation technology and building the evidence that matters today and leads us into the future.
This is again...
NANS responds to California DWC order to discontinue coverage of non-opioid...
The North American Neuromodulation Society (NANS) has issued a response to the California Division of Workers’ Compensation (DWC) order which adopts regulations to update the evidence-based...
Flexible battery could revolutionise implanted devices
Experts at Queen’s University Belfast, Belfast, UK, have designed a flexible battery that could provide an alternative to the rigid batteries that usually power...
Marking 50 years of spinal cord stimulation
The year 2017 marks the 50th anniversary for spinal cord stimulation therapy which was first used to treat pain in 1967. To celebrate the...
CE mark for Vercise Gevia deep brain stimulation system
CE mark has been granted for the Vercise Gevia deep brain stimulation (DBS) system (Boston Scientific), a rechargeable, magnetic resonance (MR) conditional device indicated...
SureTune3 deep brain stimulation software receives Health Canada licence
Medtronic has received a Health Canada licence for SureTune3 software for deep brain stimulation (DBS). The latest innovations in the SureTune technology are designed...
CE mark approval for Stimwave’s world-first fully percutaneous spinal cord stimulator...
CE mark approval has been granted for the world’s first percutaneous injectable anchor system, SandShark. The Stimwave system is used to fixate the company’s...
Giancarlo Barolat
Over a 38-year career, Giancarlo Barolat has performed over 8000 neurostimulation procedures and has seen the field develop and change in a number of...
How do you design unbiased neuromodulation trials?
At the annual meeting of the Neuromodulation Society of the UK and Ireland (NSUKI; 3–4 November, Manchester, USA) Alan Batterham (Health & Social Care...
More research needed to show potential of neuromodulation
While much has been done in the past decade to advance the field of neuromodulation, some investigators believe only small steps have been made...
New guidance on neuromodulation therapy to be published
The International Neuromodulation Society (INS) journal Neuromodulation: Technology at the Neural Interface is publishing expert, evidence-based guidance on the safe and efficient use of neuromodulation implants in patient-centred...
FDA approves BurstDR stimulation for patients with chronic pain
The US Food and Drug Administration (FDA) has approved BurstDR stimulation, a physician-designed form of spinal cord stimulation (SCS) clinically proven to provide superior outcomes for...
Study finds deep brain stimulation a cost-effective treatment method for Parkinson’s...
It has been established that deep brain stimulation is clinically superior to medical therapy for treating advanced Parkinson’s disease. Jan B Pietzsch (Department of...
Medtronic’s StealthStation cranial software receives FDA clearance as an aid for...
Medtronic has announced US Food and Drug Administration (FDA) clearance of StealthStation cranial software as an aid for deep brain stimulation (DBS) lead placement....
ReActiv8-A sustains performance at one year in clinical trial
Mainstay Medical International has announced the one-year results from the ReActiv8-A Clinical Trial, an international, multi-centre, prospective, single arm trial for ReActiv8 in people...
Study urges earlier consideration of spinal cord stimulation in chronic pain...
A study recently published in the journal Neuromodulation suggests that earlier consideration of spinal cord stimulation in chronic pain care continuum could have a...
SCS TechTalk: Innovation Starts Here
In this Boston Scientific Educational Supplement, you will read about the pace of innovation—from delivering the first and only rechargeable spinal cord stimulation (SCS) system powered by intuitive...
Most patients with chronic trunk or limb pain experience “substantial” pain...
Twenty-four month data from a postmarket, clinical registry have found that most patients suffering from chronic pain of the trunk and/or limbs reported “substantial”...
Boston Scientific acquires Cosman Medical radiofrequency ablation manufacturer
Boston Scientific Corporation has acquired Cosman Medical a privately-held manufacturer of radiofrequency ablation systems. According to a press release, the Cosman Medical team and...
Cefaly releases revamped pocket-sized model of migraine headband
Cefaly has announced that its eponymous Cefaly device—which is a US Food and Drug Administration (FDA)-approved external trigeminal nerve stimulation device for the prevention...
Higher spinal cord stimulation frequency may reduce epileptic seizure
The ability of spinal cord stimulation to affect spike-and-wave discharges has been suggested as a means to mitigate epileptic seizures in recent literature. In...
Blue Cross Blue Shield denies coverage for high-frequency spinal cord stimulation...
Blue Cross Blue Shield has denied coverage for high frequency spinal cord stimulation in at least two states. Outposts of the federation of...
St Jude Medical launches Infinity deep brain stimulation system and directional...
St Jude Medical has launched the Infinity deep brain stimulation (DBS) system and directional DBS lead in Europe.
The system, which received CE mark approval...
CE mark granted to Mainstay Medical for ReActiv8
Mainstay Medical International has received CE mark approval for ReActiv8, its proprietary implantable neurostimulation system designed to treat disabling chronic low back pain.
According to...
Boston Scientific receives US FDA approval for Precision Montage MRI spinal...
Boston Scientific Corporation has launched the Precision Montage MRI spinal cord stimulator system after receiving approval from the US Food and Drug Administration (FDA)....
First US commercial implants of Axium DRG system announced
St Jude Medical has announced the US launch and first post-approval implants of the St Jude Medical Axium Neurostimulator System for dorsal root ganglion (DRG) stimulation...
Medtronic offer first complete portfolio of full-body MR conditional neurostimulation systems
The US Food and Drug Administration (FDA) has approved Medtronic’s Specify SureScan MRI surgical leads, which are indicated for use as part of Medtronic...
St. Jude Medical: Returning innovation to chronic pain treatment
With a series of acquisitions and recent product approvals, St. Jude Medical has further solidified its place as a leading provider of treatment options...
High-density stimulation restores efficacy in failed spinal cord stimulation therapy
By reprogramming existing spinal cord stimulation systems, investigators have been able to restore efficacy in patients who have failed spinal cord stimulation therapy, and...
Timothy Deer
Timothy Deer (Centre for Pain Relief, Charleston, USA) has recently been appointed president of the International Neuromodulation Society. He believes neuromodulation is now the...
Medtronic and Samsung to collaborate on telehealth solutions for neuromodulation patients
Medtronic and Samsung Electronics America are to begin a broad-based strategic alliance aiming to speed up the development of digital health solutions for those...
SUNBURST data demonstrates superior pain relief results for Burst Stimulation over...
St Jude Medical’s Burst stimulation can relieve chronic pain more effectively than traditional tonic spinal cord stimulation (SCS), according to the SUNBURST study. The...
LUMINA data demonstrate 70% greater low back pain relief with Precision...
Final results evaluating the Precision Spectra spinal cord stimulator system (Boston Scientific) demonstrate that the device provides more than 70% greater low back pain...
St Jude Medical launches spinal cord stimulation system in Europe
St Jude Medical has launched its Proclaim Elite spinal cord stimulation (SCS) system in Europe. According to a press release, this is the world’s...
FDA approves Proclaim Elite SCS System
St Jude Medical has announced FDA approval of the new Proclaim Elite Spinal Cord Stimulation System, the first and only upgradeable and recharge-free spinal cord stimulation (SCS)...
Medtronic TYRX Neuro Absorbable Antibacterial Envelope now available for use with...
Medtronic plc has announced the first implants of the TYRX Absorbable Antibacterial Envelope with Medtronic deep brain stimulation (DBS) systems. The implants were conducted...
St Jude Medical receives CE mark for MRI conditional labelling of...
St Jude Medical has received CE mark approval for magnetic resonance imaging (MRI) conditional labelling for the company’s Prodigy MRI chronic pain system with...
FDA clears St Jude Medical Invisible Trial System
St Jude Medical has received Food and Drug Administration (FDA) approval of the Invisible Trial System. The system, which received CE mark in June...
Alim Louis Benabid
Winner of the 2015 Breakthrough Prize in Life Science, the 2014 Lasker DeBakey Clinical Medical Research Award and the North American Neuromodulation Society (NANS)...
St Jude Medical completes acquisition of Spinal Modulation
Now complete, the acquisition of Spinal Modulation adds DRG stimulation technology to the St Jude Medical chronic pain portfolio.
Medtronic announces European approval of the first and only full-body MR...
The expanded approval for full-body MRI scans applies to all patients receiving a new system and to an estimated 13,000 people in Europe already receiving Medtronic DBS therapy.
St Jude Medical announces intent to acquire Spinal Modulation
Following the completion of this acquisition, St Jude Medical will become the only medical device manufacturer to offer radiofrequency ablation (RFA), spinal cord stimulation (SCS) and dorsal root ganglion (DRG) stimulation therapy solutions for the treatment of chronic pain.