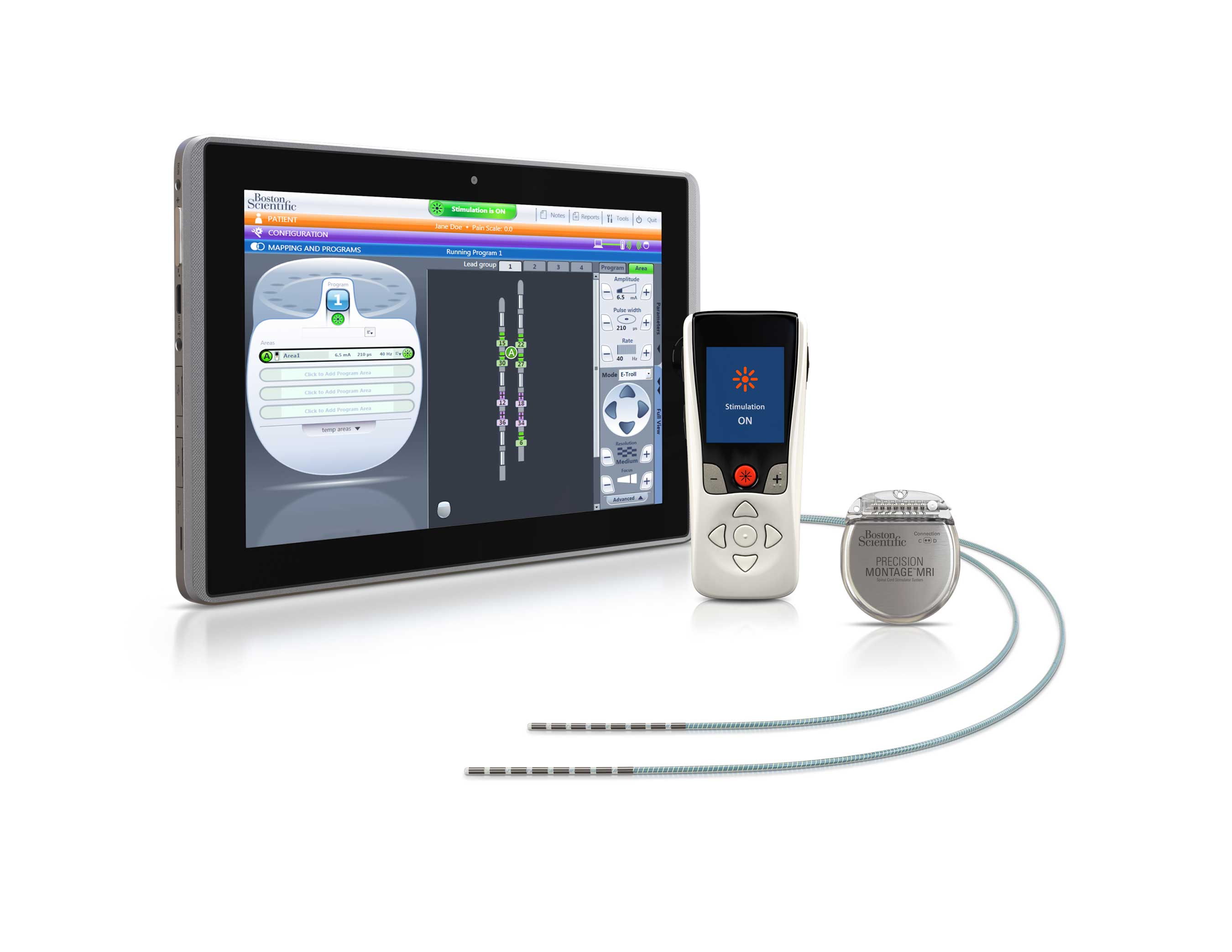
Boston Scientific Corporation has launched the Precision Montage MRI spinal cord stimulator system after receiving approval from the US Food and Drug Administration (FDA).
The Precision Montage System is designed to offer customised relief to patients with chronic pain while also enabling safe access to full body magnetic resonance imaging (MRI) in a 1.5 Tesla environment when conditions of use are met. Boston Scientific introduced the system at the 8th World Congress of the World Institute of Pain in New York City on 20-23 May, 2016.
The new spinal cord stimulation system uses the Illumina 3D algorithm—a three-dimensional, anatomy-driven computer model—designed for simple point-and-click pain targeting to support physicians in treating chronic pain.
“The Boston Scientific Illumina 3D portfolio allows me to tailor therapy for each patient consistently and effectively,” says Salim Hayek, program director, Anesthesiology Pain Medicine, University Hospital Case Medical Center, Cleveland, USA.












