SPR Therapeutics have announced the study results of its SPRINT peripheral nerve stimulation (PNS) system in the treatment of post-amputation pain.
The study demonstrated that short-term PNS therapy may provide enduring clinically significant pain relief, reduce opioid use and improve quality of life in patients with post-amputation pain, a type of neuropathic pain. This is the first study of an FDA-cleared PNS system to demonstrate statistically significant and clinically meaningful improvements in pain relief for both residual limb pain and phantom limb pain. The study, “Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Neuropathic Post-Amputation Pain: A Multi-Center Randomized Placebo-Controlled Trial” has been published in Regional Anesthesia and Pain Medicine, the official peer-reviewed publication of the American Society of Regional Anesthesia and Pain Medicine (ASRA).
Study participants had experienced post-amputation pain for periods ranging from four months to more than 30 years. All participants underwent the minimally-invasive ultrasound-guided placement of SPR’s MicroLeads which are constructed using a 100-micron wire. Participants were randomised to receive SPRINT PNS therapy or placebo for four weeks. The placebo group then crossed over and all study participants received PNS for four subsequent weeks. A study participant was considered a success if they reported significant pain relief of at least 50% during the first four weeks.
The study demonstrated that a significantly greater proportion of the participants who received SPRINT PNS experienced significant pain relief (58%) compared to 14% of those who received placebo. The delivery of therapy for four additional weeks in the SPRINT PNS group resulted in 67% of the participants reporting significant pain relief. While prospective follow up is ongoing, of those participants who completed follow-up at 12 months, 80% reported continued relief with an average pain reduction of 76%. Furthermore, opioid use among moderate to high users (which represented about a quarter of the participants) decreased by more than 70% in the SPRINT PNS group compared to less than one percent in the placebo group. The most common side effect was skin irritation or redness at the lead exit site or related to the use of adhesive patches or bandages. There were no serious side-effects.
Christopher Gilmore of the Center for Clinical Research in Winston-Salem, USA, and principal author, stated: “Post-amputation pain has traditionally been one of the most challenging neuropathic conditions we treat as interventional pain management specialists. The data clearly demonstrate that the SPRINT PNS system is a safe and effective treatment option for post-amputation pain, with the potential to produce pain relief that endures well beyond the 60-day therapy period, after which the leads are withdrawn. Given the durability of pain relief we’re observing with SPRINT, many patients will appreciate that a permanently implanted system may be avoided. The non-opioid and minimally-invasive nature of SPRINT also bodes well for its use early in the care continuum for other painful neuropathic conditions.”
According to Paul Bernacchio, president of the National Amputation Foundation, “The study results reported by the SPR Therapeutics team are great news to the amputee and Veteran communities we represent. We are in desperate need of more effective, less invasive early interventions to address the significant issue of post-amputation pain. The enduring effects of this short-term therapy and its minimally-invasive nature make it a very compelling therapy for those suffering post-amputation pain.”
“SPR Therapeutics is very appreciative of the support of our physician investigators and sincerely grateful to our study volunteers,” said Maria Bennett, CEO of SPR Therapeutics. “This latest study validates the durable results being achieved with our SPRINT PNS system. We continue to work with pain specialists on future studies as we further advance the growing body of evidence for SPRINT.”
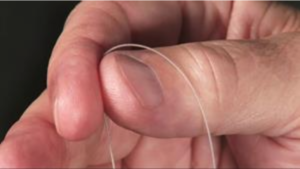
SPR Therapeutics SPRINT PNS system MicroLead:
The SPR Therapeutics’ SPRINT Peripheral Nerve Stimulation (PNS) System MicroLead is constructed using a tiny 100-micron wire, about the size of a human hair. The leads are placed by a physician during an outpatient procedure without surgery, incisions, tissue destruction or anaesthesia, and are connected to a wearable stimulator that delivers stimulation for up to 60 days of therapy, after which the leads are withdrawn.