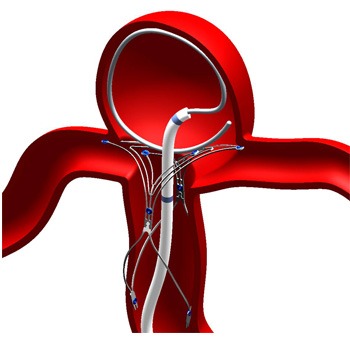
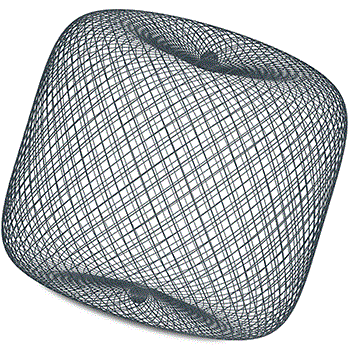
Tag: intracranial aneurysm
Finding space and maintaining shape: European physicians discuss first experiences with...
This advertorial is sponsored by WallabyPhenox.
While the endovascular treatment of intracranial aneurysms has evolved substantially in recent times and a plethora of novel technologies...
Penumbra receives European CE mark for SwiftPAC neuroembolisation coils
Penumbra has secured CE-mark approval for its SwiftPAC neuroembolisation coil, which is part of the company’s Swift coil system. SwiftPAC coils are now commercially...
US Brain Aneurysm Foundation announces 2025 research grant recipients
The US Brain Aneurysm Foundation (BAF) has announced its 2025 research grant recipients, recognising 14 researchers for their “exceptional initiatives” aimed at saving lives...
ROAR-DNA study seeks to lay foundation for UK’s first brain aneurysm...
A landmark research initiative attempting to lay the foundation for the UK’s first genetic screening programme for brain aneurysms—potentially reshaping how the country’s National...
Multidisciplinary collaboration receives US$144,500 grant to develop tool for predicting brain...
A “historic” global collaboration bringing together cutting-edge artificial intelligence (AI), neurotechnology and neuroscience has been awarded a US$144,500 grant to launch the “world’s first”...
Researchers introduce model for predicting post-coiling aneurysm recanalisation with “high discriminative...
A research team in Japan has outlined a newly developed model for predicting the likelihood of intracranial aneurysms recanalising after coil embolisation treatments. Detailing...
Galaxy Therapeutics completes enrolment of primary cohort in pivotal SEAL IT...
Galaxy Therapeutics has today announced completed enrolment of the primary cohort of patients in its SEAL IT investigational device exemption (IDE) trial.
The trial's primary...
Brain Aneurysm Foundation issues rallying cry to boost research funding for...
The Brain Aneurysm Foundation (BAF) has been joined by advocates from more than two dozen US states to support ‘Ellie's Law’—a bipartisan bill recently reintroduced in...
Endovascular treatment of brain aneurysms—is under 5mm a ‘go’ or ‘no-go’...
In light of recently presented and generally positive findings from the COAST study, which deemed coiling to be safe in the treatment of small...
Hereditary Brain Aneurysm Support achieves registered charity status
Hereditary Brain Aneurysm (HBA) Support—a patient-centred organisation providing support and resources for those affected by hereditary brain aneurysms—has announced its new status as a...
COATING study results set to have “tremendous impact” on intracranial aneurysm...
This advertorial is sponsored by WallabyPhenox.
Ahead of the COATING study’s much-anticipated final analyses, principal investigator Laurent Pierot (Reims University Hospitals, Reims, France) sits down...
COAST is clear: study finds coiling safe in small brain aneurysms
Coil embolisation of smaller intracranial aneurysms—those less than 5mm in size—has been found to be a safe approach, as per data presented for the...
Data analyses reveal possible link between gastrointestinal syndromes and brain aneurysm...
There is a potential connection between a diagnosis of certain gastrointestinal (GI) syndromes, and the formation and rupture of intracranial aneurysms, according to research presented...
Microvention announces US availability of LVIS EVO intraluminal support device
Microvention, a wholly owned subsidiary of Terumo Corporation, announced today that the LVIS EVO intraluminal support device is now commercially available for the treatment...
Conformability, softness, simplicity and simulation enable Artisse to ‘personalise’ treatments in...
This advertorial, intended for readers outside the USA only, is sponsored by Medtronic.
In light of the official European launch of the Artisse aneurysm embolisation...
ARISE I recommends multidisciplinary approach to managing brain aneurysms, chronic SDHs...
Three papers published in the journal Stroke have highlighted multidisciplinary care and global, collaborative efforts as “paramount” in the management of intracranial aneurysms, chronic...
Shining a light on hereditary brain aneurysms
Intracranial aneurysms are a largely underestimated condition, for which global awareness and clinical research are currently lacking—and these sentiments apply even more pertinently when...
“The third way” in intrasaccular aneurysm treatment demonstrates positive one-year outcomes
A device labelled “the third way” to potentially treat intracranial aneurysms via an intrasaccular approach has demonstrated positive effectiveness and safety results at one...
Imperative announces FIH study of device enabling single antiplatelet therapy after...
Imperative Care has announced the initiation of a first-in-human (FIH) clinical study for its novel stent system designed to only require single antiplatelet therapy...
Understanding brain aneurysms: Prevalence, burden, and empowering patients
In a guest piece for NeuroNews, Arūnė Simanavičienė (Kaunas, Lithuania) and Audrius Širvinskas (Vilnius, Lithuania) highlight the work being done by the Brain Aneurysm...
Current intracranial aneurysm literature reflects “a failure of the peer-review process”
A systematic review and meta-analysis of 1,356 studies and more than 410,000 patients have found that “methodological flaws and incomplete reporting” occur frequently in...
Nautilus represents “next evolution” of intrasaccular systems in aneurysm care
The Nautilus system (Endostream Medical)—a self-conforming intrasaccular flow diverter intended for the treatment of intracranial aneurysms—heralds the “next evolution” in these types of neurovascular...
US Brain Aneurysm Foundation announces 2023 research grants
The Brain Aneurysm Foundation (BAF) has announced the recipients of its 2023 research grants, providing support to academic researchers studying the underlying biology of aneurysms or...
Brain Aneurysm Foundation launches ‘Stop the Pop’ campaign
The Brain Aneurysm Foundation (BAF) has announced the launch of ‘Stop the Pop’—a new campaign intended to increase awareness of the prevalence and impact...
Continued improvement of flow diverters and intrasacculars holds key to truly...
Laurent Pierot’s (Reims University Hospitals, Reims, France) long career in the treatment of intracranial aneurysms predates not only what many consider to be the...
Rapid Medical announces new data showing “significant advantages” of Comaneci for...
Rapid Medical has announced new clinical data demonstrating the “significant advantages” of its novel Comaneci embolisation assist device over established techniques to treat ruptured...
Flow-diverter performance “comparable” between PComAs and other supraclinoid aneurysms
The concluding findings of a systematic review and meta-analysis published in the journal Neurosurgery indicate that flow-diverter performance in posterior communicating artery (PComA) aneurysms...
Researchers uncover ‘mutant origin’ of intracranial aneurysms and report promising drug...
Researchers at the RIKEN Center for Brain Science (CBS) in Wako, Japan have discovered a set of related mutations that lead to intracranial aneurysms....
Pipeline Flex embolisation device deemed safe and effective in complex intracranial...
A retrospective study from China has concluded that the Pipeline Flex embolisation device (Medtronic) is safe and effective in the treatment of complex intracranial...
WEB 17 device maintains efficacy, safety across ruptured and unruptured aneurysms
The Woven EndoBridge (WEB) 17 device (Microvention/Terumo Corporation) has demonstrated positive efficacy and safety outcomes in the prospective, multicentre CLEVER study, indicating its utility...
Systematic review queries how far PPTA aneurysm management is along “the...
A systematic review and analysis published in the journal Interventional Neuroradiology has examined how far along “the right path” management paradigms for persistent primitive trigeminal artery...
Stryker completes acquisition of Cerus Endovascular
Stryker announced today that it has completed the acquisition of Cerus Endovascular—a medical device company engaged in the design and development of neurointerventional devices...
Stent-assisted coiling fails to show superiority versus coiling alone in unruptured...
A randomised controlled trial (RCT) involving more than 200 patients has indicated that stent-assisted coiling (SAC) is not superior to coiling alone in unruptured...
Recent addition to European flow diverter market performs well in multicentre...
A recent addition to Europe’s flow diversion market—the Derivo 2 embolisation device (DED2; Acandis)—has demonstrated promise in a small-scale, multicentre study, providing a safe...
Researchers develop injectable biomaterial with potential in endovascular aneurysm treatment
Researchers in the USA have developed an injectable, ‘toothpaste-like’ biomaterial that may offer promise in the treatment of intracranial aneurysms as an alternative to...
RAAS inhibitors shown to reduce risk of intracranial aneurysm rupture in...
A multicentre study of more than 3,000 people with high blood pressure and brain aneurysms found that the use of renin-angiotensin-aldosterone system (RAAS) inhibitors—a...
Latest data reinforce evidence on safety and effectiveness of Contour intra-aneurysmal...
Fresh data from the CERUS study—the first multicentre clinical trial evaluating safety and effectiveness outcomes with the Contour neurovascular system (Cerus Endovascular)—have indicated “encouraging...
Wallaby Medical launches Avenir coil system for haemorrhagic stroke treatment in...
Wallaby Medical has partnered with Japan Lifeline (JLL) to bring its Pharmaceuticals and Medical Devices Agency (PMDA)-approved Avenir coil system to the Japanese market....
Large prospective study demonstrates high efficacy and low mortality rates with...
The Diversion-p64 study—the largest prospective study using the p64 flow modulation device (phenox) to date—has demonstrated that the device has a high level of...
Medtronic recalls Pipeline Flex embolisation devices over risk of delivery system...
Medtronic has issued a recall of its Pipeline Flex embolisation device and Pipeline Flex embolisation device with Shield technology products due to a risk...
ESMINT 2021: COATING study “badly needed” to assess surface modification of...
This year’s Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT 2021; 8–10 September, Nice, France and virtual) saw details of the...
“Welcome and exciting”: Evidence mounts in favour of flow diversion technologies
Multiple pieces of clinical research supporting the use of flow diverter devices to treat intracranial aneurysms have come to light so far in 2021....
Fluid Biotech raises US$4.7 million in oversubscribed seed funding round
Fluid Biotech, a medical device start-up company based in Calgary, Canada, has announced the completion of an oversubscribed seed fundraising round totalling US$4.7 million—and...
FRED flow diverter safe and effective in treatment of “appropriately selected”...
The Flow Redirection Endoluminal Device stent system (FRED; MicroVention)—a flow diverter used to treat intracranial aneurysms—has been deemed safe and effective for this indication,...
Data from 1,000 aneurysms indicate Pipeline Embolization Device is safe and...
The endovascular treatment of intracranial aneurysms using the Pipeline Embolization Device (PED; Medtronic) has been deemed safe and effective following a study involving data...
Perflow Medical announces first clinical use of Stream17 Dynamic Neurothrombectomy Net
Perflow Medical has announced the first successful clinical use of its Stream17 Dynamic Neurothrombectomy Net—a lower profile device designed to effectively treat haemorrhagic and...
Stryker’s Neuroform Atlas stent approved to treat aneurysms of the posterior...
Stryker announced that it has received US Food and Drug Administration (FDA) approval for an expanded indication of its Neuroform Atlas stent system as...
LIVE from SNIS: Treatment advancements have reduced mortality rates from unruptured...
Mortality rates after treatment of unruptured intracranial aneurysms have substantially decreased in the past decade, according to new findings presented at the Society of...
phenox launches p64 MW Flow modulation device with HPC coating technology
phenox has announced the European launch of the new p64 MW Flow modulation device, intended for the treatment of intracranial aneurysms. The device received...
Cerus Endovascular’s 021 Contour neurovascular system approved in Europe
Cerus Endovascular has received CE Mark approval for its 0.021-inch Contour neurovascular system, compatible with smaller commercially available 021 microcatheters for the treatment of...
Cerus Endovascular receives CE mark for its Neqstent Coil Assisted Flow...
Cerus Endovascular has announced that it has received CE mark approval for its Neqstent Coil Assisted Flow Diverter device. The flow diverter is designed...
Cerus Endovascular receives CE mark approval for its Contour Neurovascular System...
Cerus Endovascular has received CE mark approval for its lead product, the Contour Neurovascular System, for the treatment of intracranial aneurysms. The system incorporates...
MicroVention’s FRED flow diverter now FDA approved
MicroVention, a US based subsidiary of Terumo and a global neurovascular company announced the US FDA premarket approval (PMA) for the FRED (Flow re-direction...
Perflow Medical receives CE mark approval of Cascade Agile
Perflow Medical has announced they have received CE Mark approval for the Cascade Agile non-occlusive remodelling net (Cascade Agile). Expanding the Cascade product family,...
Phenox announces exclusive distribution agreement with Wallaby Medical for the Avenir...
Phenox announces that it has signed an exclusive distribution agreement with Wallaby Medical for commercialisation of the Avenir Detachable Coil System in the US...
ESMINT 2019: Drugs to strengthen aneurysm wall will be the future,...
Mika Niemelä (Helsinki, Finland) talks to BLearning Neuro about the ‘Decision making in unruptured aneurysms’ session he chaired at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy;...
Three-year outcomes of the Surpass system confirm its safety and efficacy
Three-year outcomes of the Surpass intracranial aneurysm embolisation system pivotal trial to treat large or giant wide neck aneurysm (SCENT trial) confirm the safety...
Stryker gains US FDA premarket approval for Neuroform Atlas stent system
Stryker has announced the premarket approval (PMA) of the Neuroform Atlas stent system by the US Food and Drug Administration (FDA).
Neuroform Atlas—according to the...
First patients enrolled in CERUS study to treat intracranial aneurysms using...
Cerus Endovascular has announced enrolment of the first patients in the CERUS study (Contour Neurovascular System European Pre-Market Unruptured Aneurysm Study). The CERUS study...
Acandis receives European CE mark approval for its ACCERO stent
Acandis has announced that it has received European CE mark approval for its ACCERO stent, which is now available for sale in Europe. The...
First intracranial aneurysm patients treated with BRAVO Flow Diverter
Cerenovus, part of the Johnson & Johnson Medical Devices Companies, has announced that the first patients have been treated with the BRAVO Flow Diverter,...
FDA gives HDE approval for Neuroform Atlas to treat wide neck...
The US Food and Drug Administration has approved the Neuroform Atlas Stent System (Stryker) for marketing under a humanitarian device exemption (HDE). The device...
Cerus Endovascular provides corporate update, announces first-in-man use of intra-saccular stent
Cerus Endovascular has provided an update on its lead development programmes, including the Contour Neurovascular System as well as the NEQSTENT Intra-Saccular Stent.
“The past...
Coiling of <5mm aneurysms safe and efficacious in Target registry sub-analysis
Based on a sub-analysis of the data from the prospective Target aneurysm multicentre registry, the coiling of aneurysms <5mm in size with Target coils...
Contour Neurovascular System receives CE mark approval
The Contour Neurovascular System (Cerus Endovascular) has received CE mark approval for the treatment of intracranial aneurysms. Contour is a unique, fine mesh braid...
First patients treated in study of Contour system
The first patients have been enrolled and treated in a prospective, single-arm, multicentre study evaluating the Contour Neurovascular System (Cerus Endovascular). Used for the...
Better neck coverage with “bulged centre” of Barrel device in wide-necked...
A single-centre study reports that stent-assisted coiling with the Barrel stent (Medtronic) is a feasible, safe, and efficient method for embolisation of intracranial wide-necked...
Hydrogel coils beat bare platinum in comparison study
The GREAT randomised controlled trial has confirmed that better outcomes can be achieved with hydrogel coils compared to bare platinum coils for the treatment...
Utility of patient-specific neurovascular models in neurointerventional training
By Andreas MJ Frölich
Endovascular aneurysm therapy today is characterised by a rapidly growing and evolving collection of available treatment devices. The challenge in achieving...
Acclino stents feasible in complex bifurcation aneurysms at mid-term follow-up
A new study has found stent-assisted endovascular techniques with Acclino stents (Acandis) are a feasible treatment for complex intracranial aneurysms. Reporting in the Journal...
Medina embolisation device represents “a major step forward” in aneurysm treatment
According to a report of a single centre’s experience using the Medina embolisation device (Medtronic) for the treatment of unruptured aneurysms, the device “represents...
High rate of aneurysm occlusion with p64 at mid- and long-term...
Investigators using the p64 Flow Modulation Device (Phenox) for the treatment of unruptured intracranial aneurysms at six centres have revealed that mid- and long-term...
ANSWER study for wide-neck aneurysms meets primary endpoints
In the ANSWER (Adjunctive neurovascular support of wide-neck aneurysm embolization and reconstruction) study of the PulseRider device (Pulsar Vascular), enrolment, 30-day and 180-day follow-up...
Medina Embolization Device and Pipeline Shield: LINNC Wrap Up Interviews
Find the latest updates from Prof Monika Killer and Dr Saleh Lamin following this year’s LINNC Congress in Paris. We consider our latest technologies,...
New aneurysm device acts as both flow disruptor and diverter
The Contour Neurovascular System (Cerus Endovascular) will offer a new option for treating intracranial aneurysms. According to Kyriakos Lobotesis, Principle Clinical Advisor at Cerus,...
Terumo acquires Sequent Medical
Terumo Corporation has announced that it has entered into an agreement to purchase Sequent Medical, known for its WEB aneurysm embolisation device.
The purchase price...
Medina Embolization Device
Medina™ is designed as an intra-saccular flow diverter, diverting flow away from the aneurysm sac and through the parent artery by forming a mesh scaffold...
Penumbra Coil 400 safe and effective in small aneurysms
The results of a multicentre study have shown that catherisation with the larger profile coil delivery microcatheter and aneurysm occlusion with large volume coils...
Sequent Medical initiates study of WEB aneurysm embolisation system for ruptured...
Sequent Medical has begun enrolling patients in a study which will evaluate the safety and effectiveness of the WEB (woven endobridge) aneurysm embolisation system...
First coil key during PulseRider procedures in wide-neck aneurysms
At the 2015 annual meeting of the European Society of Minimally Invasive Neurological Therapy (ESMINT; 10–12 September, Nice, France) Gyula Gal (Department of Radiology,...
Almost no learning curve with Barrel reconstruction device
The Barrel Vascular Reconstruction device (Medtronic) is one of the newest offerings for the treatment of intracranial bifurcation aneurysms. Since receiving the CE mark...
Enrolment complete for PulseRider US clinical trial
Pulsar Vascular has announced that they have reached the target enrolment in their clinical trial for PulseRider device for the treatment of intracranial aneurysms....
New device is “equivalent to, or better than” known flow diverters...
In the first study to be published reporting on endovascular aneurysm treatment with the p64 flow modulation device (Phenox), Francesco Briganti and colleagues have found that the device provides a safe procedure with no technical complications.
Phenox launches pToWin study to assess efficacy of pCONus bifurcation aneurysm...
Phenox GmbH has announced the start of the pToWin study after enrolling the first patient on 2 September, 2015. The prospective, multicentre, single-arm study...
Medtronic acquires Medina Medical
Medtronic plc has announced that it has acquired Medina Medical, a Menlo Park, California, USA-based and privately-held medical device company focused on commercialising state-of-the-art...
Radiation reduced with flow diversion for treatment of internal carotid artery...
According to new research, treatment of large and giant proximal internal carotid artery aneurysms using the Pipeline embolisation device (Covidien/Medtronic) requires less radiation, less...
Sequent Medical announces long-term clinical data and commercial release of its...
Sequent Medica has announced the presentation of prospective long-term clinical data for the WEB aneurysm embolisation system at the Societe Francaise de Neuroradiologie (SFNR)...
Next generation Medina coil system shows improvements in early human experience
In early use of the Medina coil system, researchers have found the device to be a next generation coil that combines the familiar procedural safety and technique associated with conventional coils, with improved circumferential aneurysm filling, which, they say, it is thought will lead to improved long-term outcomes.
FDA approves Pipeline Flex embolisation device
Designed to divert blood flow away from an aneurysm, the Pipeline Flex embolisation device (Medtronic) features a braided cylindrical mesh tube that is implanted across the base or neck of the aneurysm.
PulseRider safe and effective in early USA experience
Initial experience with PulseRider (Pulsar Vascular) has shown the device to be safe and effective as an adjunct in the treatment of bifurcation aneurysms arising at the basilar apex or carotid terminus.
Medina Medical announces CE mark for its embolisation coil
Medina Medical has announced that it has received CE mark authorisation for its Embolization Framing Coil for commercial distribution in the European Union.
Codman Neuro gains exclusive rights to market and promote PulseRider in...
PulseRider is a minimally invasive device intended for use with embolic coils in the treatment of unruptured wide-neck intracranial aneurysms originating on or near a bifurcation.
FDA IDE approval for PulseRider
The IDE allows Pulsar Vascular to begin a multicentre clinical trial in support of a humanitarian device exemption (HDE) to evaluate the PulseRider for US approval for wide neck aneurysms at or near a bifurcation of the basilar tip or carotid terminus.
Pulsar Vascular gets CE mark for Pulserider
Pulsar Vascular announced that it has received European CE mark approval for Pulserider. This unique implant is used to bridge the neck of cerebral aneurysms previously not amenable to endovascular therapy. This new device is implanted via standard, minimally invasive, endovascular techniques thus providing an alternative treatment option to open surgery.
Phenox receives CE mark approval for pCONus Bifurcation Aneurysm Implant
The pCONus implant for treatment of complex, wide neck intracranial bifurcation aneurysms has been granted the CE mark for commericalisation in Europe.
Phenox receives CE mark approval for p64 Flow Modulation Device
The p64 Flow Modulation Device is an intraluminal flow diverter for treatment of complex intracranial aneurysms and dissections.