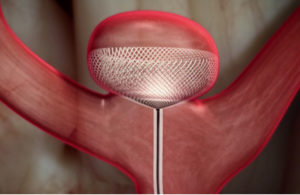
Cerus Endovascular has announced enrolment of the first patients in the CERUS study (Contour Neurovascular System European Pre-Market Unruptured Aneurysm Study). The CERUS study aims to assess the safety of the company’s Contour Neurovascular System, which is designed to reduce the risk of rupture when treating patients with intracranial aneurysms.
CERUS is a prospective, 30-patient, single-arm, multicentre, pre-market trial. The study is expected to complete enrolment within the next four months and is being conducted at 10 leading European neurological centres in Germany, France, Austria and Denmark. Co-principal investigator, Professor Thomas Liebig, of Ludwig Maximillian’s University Hospital, Munich, has performed three of the first four cases with the fourth performed at University Hospital Schleswig-Hostein, Kiel, in Germany.
When asked about his impression, Liebig said, “The Contour System combines the benefits of flow disruption and redefinition of the aneurysm-to-parent vessel-border without any material in the parent artery. Thus, it doesn´t mandate long-term antiplatelet therapy. Sizing was straightforward in these first cases and was done with regard to the neck only since the Contour does not aim for bulk replacement of the aneurysmal cavity. Angulation and irregularity of the aneurysm dome seemed to play a lesser role, as well. We are aware of the need for a more valid database with more cases to support these impressions but at the moment we are quite content with our initial experience and look forward to the co-investigators experiences and to the first control visit of the patients we have treated so far.”
The Contour Neurovascular System is a next generation intra-saccular flow diverter and flow disruptor which targets the neck of the aneurysm. Intra-saccular flow diverters divert blood flow from the aneurysm and promotes healing, thereby reducing the risk of aneurysm rupture, a main cause of haemorrhagic stroke. The device is easily positioned and can be repositioned should more optimal device positioning be warranted. Accurate positioning of the device and effective delivery and deployment are important factors in the successful treatment of intracranial aneurysms. After deployment, the device conforms to the wall of the lower hemisphere of the aneurysm and across the neck sealing the neck opening.













