
The first patients have been enrolled and treated in a prospective, single-arm, multicentre study evaluating the Contour Neurovascular System (Cerus Endovascular). Used for the treatment of intracranial aneurysms, the Contour System is an intrasaccular, self-anchoring, embolisation device targeting the aneurysm neck.
These first cases were recently presented at the ABC-WIN Seminar (15–20 January, Val d’Isère, France) by the study principal investigator, Kyriakos Lobotesis (Imperial College NHS Healthcare Trust, London, UK).
The study is including patients with unruptured saccular intracranial aneurysms in both the anterior and posterior circulation. Patients with ruptured aneurysms or aneurysms that have been previously treated with other devices are excluded from this specific study.
According to Lobotesis, the Contour system lends itself to all types of aneurysms but, in particular, wide-necked challenging bifurcation aneurysms. The shape of the device across the neck, he said, provides a natural carina dividing and diverting flow away from the sac and into the branching vessels.
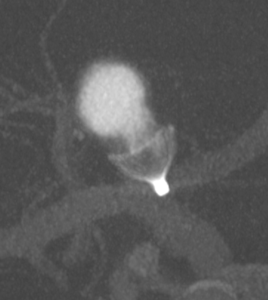
Speaking to NeuroNews, Lobotesis described his experience with the device in the study: “One of the most notable cases to date was a patient where the device, following MHRA approval here in the UK, was used on compassionate grounds. This was discussed in some detail at ABC-WIN and comprised of a large wide-necked MCA (middle cerebral artery) aneurysm that had initially recurred following coiling and subsequently recurred following WEB (MicroVention) compaction. The Contour in this case was elegantly deployed across the neck of the aneurysm proximal to the invaginated/collapsed WEB and compacted coils. The recurrence from the WEB was shallow, however the Contour’s unique shape and features allowed it to be positioned perfectly without any significant problems.”
He added that in the study so far there have been no complications to date: “All devices in the trial have behaved as planned, with predictable and stable deployment followed by stasis and elimination of the aneurysm at the end of the procedure. All patients were discharged according to plan with no adverse events.”
The current study is a multicentre European study with five clinical sites currently approved and enrolling patients. Additionally, with CE mark pending, multiple other centres across Europe are due to come on-board imminently as part of a post-market surveillance study.













