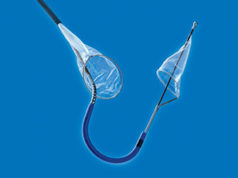
Innovative Cardiovascular Solutions (ICS) has completed an oversubscribed Series B round of financing totalling US$5 million. The company is currently developing the EMBLOK Embolic Protection System which is designed for use in transcatheter aortic valve implantation (TAVI) and other structural heart procedures where embolic protection is required. Proceeds will be used for device development and initiation of a 510(k) clinical study in the USA.
“ICS has made tremendous progress in the past 12 months with the initiation of human clinical cases in Milan, Italy. At the halfway mark of study enrolment, initial results look promising,” says R Kevin Plemmons, ICS’s co-founder and CEO. “We are pleased by the strong vote of confidence from our investors supporting the pursuit of this innovative and comprehensive approach to embolic protection.”
“The EMBLOK system is intuitive and easy to use,” says Azeem Latib, San Raffaele Hospital in Milan, Italy. “It is the only system to provide full capture and protection from embolic material released during TAVI and has become a necessary addition in my left heart interventions.”
According to a company release, the EMBLOK system is the world’s first and only embolic protection device designed to offer complete circumferential aortic collection while protecting the cerebral, abdominal and peripheral vasculature from liberated embolic debris. In addition, the EMBLOK system features an integrated 4F radiopaque pigtail catheter which provides the physician constant visualisation, while eliminating the need for unnecessary dye injections to verify positioning. The entire system is 11F and allows two devices (i.e., embolic filter and pigtail catheter) to be deployed through a single femoral puncture site.