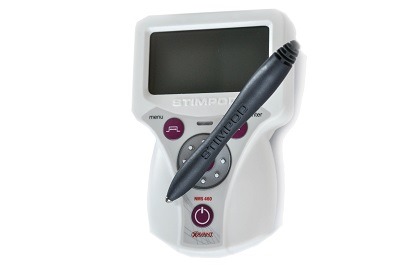
 The US Food and Drug Administration (FDA) has cleared the Stimpod NMS460 (Xavant Technology). With the US patent awarded on proprietary hybrid pulsed radio frequency (PRF) waveform, this non-invasive neuromodulation device is focused on the symptomatic relief and management of chronic intractable pain, as well as an adjunctive treatment in the management of post-surgical pain, post-traumatic acute pain problems, and an adjunct for pain control due to rehabilitation.
The US Food and Drug Administration (FDA) has cleared the Stimpod NMS460 (Xavant Technology). With the US patent awarded on proprietary hybrid pulsed radio frequency (PRF) waveform, this non-invasive neuromodulation device is focused on the symptomatic relief and management of chronic intractable pain, as well as an adjunctive treatment in the management of post-surgical pain, post-traumatic acute pain problems, and an adjunct for pain control due to rehabilitation.
According to the company, the current standard of care for chronic pain includes drug cocktails such as Corticosteroids, Opiate pain relievers, injections, and beyond combined with treatments like physical therapy and counselling. Treatments can come with various short-term and long-term side effects.
The Stimpod NMS460, however, is a non-invasive, non-drug solution with a fast onset of effect at a fraction of the cost of comparable treatments, states Xavant Technology in a press release. The device applies its patented PRF waveform to the affected area transcutaneously. This waveform creates electromagnetic effects similar to invasive pulsed radio frequency treatments, and several case studies have shown instant and dramatic relief of chronic intractable pain. The Stimpod NMS460 also incorporates the nerve-locating technology which features a nerve mapping probe that enables practitioners to locate nerves and evaluate the treatment progress of damaged nerves.
“We are thrilled at the news that our revolutionary device can now be used in the USA,” says Corlius Birkill, CEO of Xavant Technology. “This groundbreaking technology has the ability to help tens if not hundreds of millions of people just in the USA as a valuable treatment asset for neurologists, chiropractors, acupuncturists, physical therapists, physiatrists, and medical pain practitioners.”













