Tag: flow diverter
“It’s quite a revolution”—COATING investigators discuss study’s initial findings and wider...
This advertorial is sponsored by WallabyPhenox.
Following the late-breaking presentation of new data from the global COATING trial comparing two endovascular treatment approaches for unruptured...
Latest Pipeline Vantage device produces “acceptable outcomes” in unruptured aneurysms
A new study, published in the Journal of NeuroInterventional Surgery (JNIS) late last year, has assessed the safety of the latest iteration of the...
Pipeline Flex embolisation device deemed safe and effective in complex intracranial...
A retrospective study from China has concluded that the Pipeline Flex embolisation device (Medtronic) is safe and effective in the treatment of complex intracranial...
LuSeed Vascular appoints Robert Stern to board of directors
LuSeed Vascular has announced that Robert Stern, an experienced medical device executive and investor, has been nominated as the vice chairman of the company’s...
SNIS 2022: WEB flow diverter shown to be safe and effective...
The Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July 2022, Toronto, Canada) saw research involving the Woven EndoBridge (WEB; Microvention/Terumo) device disclosed for the...
Silk Vista Baby demonstrates safety and effectiveness in PICA aneurysm treatment
Following a retrospective review of databases from multiple different centres, researchers have reported the safe and effective use of the Silk Vista Baby (Balt)...
Microvention announces treatment of first US patient with FRED X flow...
Microvention, a subsidiary of Terumo, has announced the first US clinical case using its next-generation flow diverter, the FRED X device, at Thomas Jefferson University Hospital...
Evasc Neurovascular announces enrolment of first patient in French eClips study
Evasc Neurovascular has announced enrolment of the first patient in its eClips safety, feasibility and efficacy study (EESIS-FR) in France. The study will be...
Medtronic recalls Pipeline Flex embolisation devices over risk of delivery system...
Medtronic has issued a recall of its Pipeline Flex embolisation device and Pipeline Flex embolisation device with Shield technology products due to a risk...
ESMINT 2021: COATING study “badly needed” to assess surface modification of...
This year’s Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT 2021; 8–10 September, Nice, France and virtual) saw details of the...
“Welcome and exciting”: Evidence mounts in favour of flow diversion technologies
Multiple pieces of clinical research supporting the use of flow diverter devices to treat intracranial aneurysms have come to light so far in 2021....
Fluid Biotech raises US$4.7 million in oversubscribed seed funding round
Fluid Biotech, a medical device start-up company based in Calgary, Canada, has announced the completion of an oversubscribed seed fundraising round totalling US$4.7 million—and...
FRED flow diverter safe and effective in treatment of “appropriately selected”...
The Flow Redirection Endoluminal Device stent system (FRED; MicroVention)—a flow diverter used to treat intracranial aneurysms—has been deemed safe and effective for this indication,...
Flow diverter study with virtual participants “as effective as traditional clinical...
A study involving virtual participants—rather than real patients—was “as effective as traditional clinical trials” in evaluating the treatment of brain aneurysms using a flow...
Oxford Endovascular raises US$10 million to develop brain haemorrhage device
Oxford Endovascular has announced that it has raised US$10 million in a series A funding round for the development of its OxiFlow micro stent,...
Balt receives CE mark for its Silk Vista flow diverter
Balt has received CE mark approval for its Silk Vista flow diverting stent to treat patients with unruptured intracranial aneurysms, in turn enabling commercialisation...
Cerus Endovascular receives CE mark for its Neqstent Coil Assisted Flow...
Cerus Endovascular has announced that it has received CE mark approval for its Neqstent Coil Assisted Flow Diverter device. The flow diverter is designed...
Use of optical coherence tomography deemed feasible after first-in-human analysis
Results of the first-in-human analysis of acute interactions and healing process after flow diverter implantation using optical coherence tomography (OCT) imaging was presented by...
Self-expanding bioabsorbable flow-diverting stent shows a favourable safety profile
The preliminary safety data of a “self-expanding primarily bioabsorbable flow-diverting” stent has shown to be consistent with a favourable profile in terms of mechanical...
MicroVention’s FRED flow diverter now FDA approved
MicroVention, a US based subsidiary of Terumo and a global neurovascular company announced the US FDA premarket approval (PMA) for the FRED (Flow re-direction...
Surface modification: From bench to clinical practice
Demetrius Lopes has been working on the surface modification of stents and flow diverters, given that thromboembolic and antiplatelet-related complications represent the majority of...
Flow diverter outcomes are “not device, but technique dependent”
Patrick Brouwer (Stockholm, Sweden) talks to BLearning at SNIS 2019 (22–25 July, Miami, USA) about some of the issues associated with early flow diverters and why there...
Stryker launches next-generation flow diverter for treatment of brain aneurysms
Stryker has announced the availability of the Surpass Evolve flow diverter following CE-mark approval in March. Surpass Evolve is Stryker’s latest entrant into the...
Pre-market approval for Surpass Streamline flow diverter
The US Food and Drug Administration has granted pre-market approval (PMA) for the Surpass Streamline flow diverter to treat unruptured large and giant wide...
High rate of aneurysm occlusion with p64 at mid- and long-term...
Investigators using the p64 Flow Modulation Device (Phenox) for the treatment of unruptured intracranial aneurysms at six centres have revealed that mid- and long-term...
New aneurysm device acts as both flow disruptor and diverter
The Contour Neurovascular System (Cerus Endovascular) will offer a new option for treating intracranial aneurysms. According to Kyriakos Lobotesis, Principle Clinical Advisor at Cerus,...
Radiation reduced with flow diversion for treatment of internal carotid artery...
According to new research, treatment of large and giant proximal internal carotid artery aneurysms using the Pipeline embolisation device (Covidien/Medtronic) requires less radiation, less...
FDA approves Pipeline Flex embolisation device
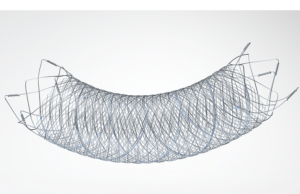
Designed to divert blood flow away from an aneurysm, the Pipeline Flex embolisation device (Medtronic) features a braided cylindrical mesh tube that is implanted across the base or neck of the aneurysm.