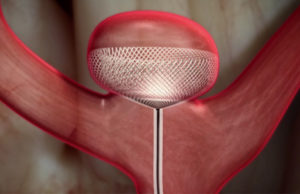
Tag: Cerus Endovascular
Stryker completes acquisition of Cerus Endovascular
Stryker announced today that it has completed the acquisition of Cerus Endovascular—a medical device company engaged in the design and development of neurointerventional devices...
Adjunctive therapy demonstrates comparable aneurysm occlusion rates to established flow diverters
The Neqstent coil-assisted flow diverter (Cerus Endovascular) has demonstrated comparable occlusion rates to those seen with other, similar flow diversion technologies, as well as...
Cerus Endovascular receives FDA clearance for 027 microcatheter products
Cerus Endovascular has received US Food and Drug Administration (FDA) 510(k) clearance for its 027 microcatheters, available in two lengths, expanding on a product...
Latest data reinforce evidence on safety and effectiveness of Contour intra-aneurysmal...
Fresh data from the CERUS study—the first multicentre clinical trial evaluating safety and effectiveness outcomes with the Contour neurovascular system (Cerus Endovascular)—have indicated “encouraging...
Cerus Endovascular announces Contour Neurovascular System™ IDE trial approval
Cerus Endovascular has announced that the US. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application to conduct a US...
Cerus Endovascular receives FDA breakthrough device designation for Contour Neurovascular System
Cerus Endovascular has received breakthrough device designation from the US Food and Drug Administration (FDA) for its Contour Neurovascular System.
The Contour Neurovascular System is...
First robotic-assisted intracranial implant of Cerus Endovascular contour intrasaccular device
Cerus Endovascular has announced in a press release the first-ever robotic-assisted intracranial implant of its Contour intrasaccular device.
The procedure was completed by Nitin Dange...
Cerus Endovascular and Balt enter strategic distribution agreement for devices treating...
Cerus Endovascular and AB Medica, a subsidiary of Balt, has announced that they have entered into a strategic distribution agreement providing AB Medica with...
CerusEndo MC 021 microcatheter wins CE mark
Cerus Endovascular has announced that it has received CE mark approval for its CerusEndo MC 021 microcatheter, designed to allow physicians to access tortuous...
Cerus Endovascular’s 021 Contour neurovascular system approved in Europe
Cerus Endovascular has received CE Mark approval for its 0.021-inch Contour neurovascular system, compatible with smaller commercially available 021 microcatheters for the treatment of...
Cerus Endovascular receives CE mark for its Neqstent Coil Assisted Flow...
Cerus Endovascular has announced that it has received CE mark approval for its Neqstent Coil Assisted Flow Diverter device. The flow diverter is designed...
Cerus Endovascular receives FDA Approval for its first microcatheter
Cerus Endovascular receives US FDA approval for its first microcatheter. The 021 microcatheter will be offered with three different distal configurations, and commercial sales...
Cerus Endovascular receives CE mark approval for its Contour Neurovascular System...
Cerus Endovascular has received CE mark approval for its lead product, the Contour Neurovascular System, for the treatment of intracranial aneurysms. The system incorporates...
First patients enrolled in CERUS study to treat intracranial aneurysms using...
Cerus Endovascular has announced enrolment of the first patients in the CERUS study (Contour Neurovascular System European Pre-Market Unruptured Aneurysm Study). The CERUS study...
Cerus Endovascular promotes Stephen Griffin to president
Cerus Endovascular has announced the promotion of Stephen Griffin to president of the company, effective immediately. Griffin had previously served as the company’s chief...
Cerus Endovascular provides corporate update, announces first-in-man use of intra-saccular stent
Cerus Endovascular has provided an update on its lead development programmes, including the Contour Neurovascular System as well as the NEQSTENT Intra-Saccular Stent.
“The past...
Contour Neurovascular System receives CE mark approval
The Contour Neurovascular System (Cerus Endovascular) has received CE mark approval for the treatment of intracranial aneurysms. Contour is a unique, fine mesh braid...
First patients treated in study of Contour system
The first patients have been enrolled and treated in a prospective, single-arm, multicentre study evaluating the Contour Neurovascular System (Cerus Endovascular). Used for the...
New aneurysm device acts as both flow disruptor and diverter
The Contour Neurovascular System (Cerus Endovascular) will offer a new option for treating intracranial aneurysms. According to Kyriakos Lobotesis, Principle Clinical Advisor at Cerus,...