Tag: Tigertriever
Refined patient selection and tailored devices revitalise thrombectomy for MeVO stroke
Late-breaking data from two randomised controlled trials (RCTs) presented at this year’s International Stroke Conference (ISC; 4–6 February, New Orleans, USA) have found mechanical...
Rapid completes enrolment in DISTALS trial evaluating Tigertriever 13 device for...
Rapid Medical has announced the completion of patient enrolment in the DISTALS trial—a multicentre randomised study assessing the Tigertriever 13 device in patients with distal/medium-vessel occlusions...
Rapid Medical announces enrolment in “first-ever” study on cognitive benefits of...
Rapid Medical has announced enrolment of the first patient in its COGNITIVE study—which, according to the company, is the first study to examine a...
Rapid Medical completes successful stroke procedures with “world’s first” robotic thrombectomy...
Rapid Medical has announced what it claims is the first successful robotic thrombectomy, in Medellín, Colombia. Two patients were treated with the robotic Tigertriever—an...
Rapid announces Japanese approval and exclusive partnership with Kaneka for Tigertriever
Rapid Medical has announced Japanese approval for its Tigertriever revascularisation device. With Pharmaceuticals and Medical Devices Agency (PMDA) approval, Tigertriever serves as “the first...
Tigertriever device produces high treatment-success rate in challenging ICAD stroke patients
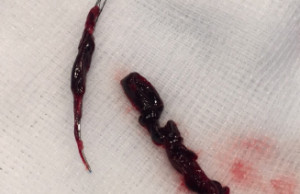
Rapid Medical has announced new data demonstrating “excellent” first-pass treatment success using the Tigertriever device in complex ischaemic stroke patients with underlying intracranial atherosclerotic disease (ICAD)....
Rapid gains Chinese NMPA regulatory approval for Tigertriever thrombectomy device
Rapid Medical has announced Chinese National Medical Product Administration (NMPA) approval for its adjustable Tigertriever revascularisation device, making it the first device to offer...
Rapid Medical gains US FDA clearance for “smallest and only” adjustable...
Rapid Medical announced at the ongoing Society of NeuroInterventional Surgery’s (SNIS) 19th annual meeting (25–29 July, Toronto, Canada) that it has recently received US...
Rapid Medical begins enrolment in DISTALS trial treating new ischaemic stroke...
Rapid Medical announced today the enrolment of the first patient in the DISTALS study, which the company claims is the first-ever US Food and...
Rapid Medical to initiate trial expanding thrombectomy treatment across distal regions...
Rapid Medical today announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for what it claims is the first ever trial...
New thrombectomy device offers promising results
In a late-breaking presentation given on the final day of the International Stroke Conference (ISC) virtual meeting (17–19 March), researchers presented a “promising” new...
Tigertriever receives FDA clearance following ISC presentation
Rapid Medical has announced the US Food and Drug Administration (FDA) clearance of its Tigertriever revascularisation device for use in the treatment of ischaemic stroke. According...
Enrolment completed early for Rapid Medical’s TIGER study for thrombectomy device
Rapid Medical has announced that it has completed enrolment in the TIGER (Treatment with Intent to generate reperfusion) study ahead of the planned scheduled....
First patient enrolled in TIGER pivotal clinical study
The first patients have been enrolled in the TIGER (Treatment with Intent to Generate Reperfusion) study. This is a multicentre study of the performance...
First patient enrolled in TIGERTRIEVER acute ischaemic stroke registry
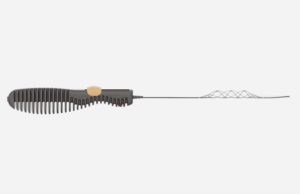
The first patient has been enrolled in the TIGERTRIEVR registry at the Cantonal Hospital of Lucerne, Switzerland. The Tigertriever (Rapid Medical) is a one-of-a-kind, fully-visible,...
Rapid Medical announces $9M in new funding
Rapid Medical has completed a Series B financing of $9M to advance the commercialisation of the company’s minimally invasive stroke treatment and prevention products, the Tigertriever...
First stroke patient treated with Rapid Medical’s Tigertriever
Rapid Medical’s Tigertriever has been used to treat its first patient. Using Rapid Medical’s proprietary technology, this stent retriever is designed to be fully...
First stroke patient treated with Rapid Medical’s Tigertriever
Using Rapid Medical's proprietary technology, this stent retriever is designed to be fully visible and controllable. The device can be adjusted by physicians to fit the dimensions of blood vessels causing acute ischaemic stroke, and is the first of its kind, according to the company.