Rapid Medical has announced Japanese approval for its Tigertriever revascularisation device. With Pharmaceuticals and Medical Devices Agency (PMDA) approval, Tigertriever serves as “the first device to offer individualised solutions for mechanical thrombectomy”, a Rapid press release claims.
Rapid Medical has announced Japanese approval for its Tigertriever revascularisation device. With Pharmaceuticals and Medical Devices Agency (PMDA) approval, Tigertriever serves as “the first device to offer individualised solutions for mechanical thrombectomy”, a Rapid press release claims.
Kaneka Corporation has exclusive distribution rights with Rapid in Japan as well, the release adds.
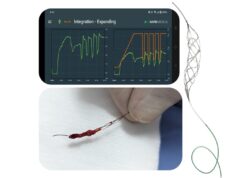
According to Rapid, Tigertriever technology is inspired by advancements in aerospace engineering, and “transforms” thrombectomy procedures from a passive to an active approach.
Contrasting with traditional stent retrievers, Tigertriever offers added user control—something that is of particular importance in Japan and other Asian countries with a high prevalence of underlying intracranial atherosclerotic disease (ICAD).
The device can be expanded and reduced “on demand”, activating clot integration and potentially reducing the risk of vessel perforation or damage during device retrieval, according to Rapid.
“Japan now has access to a single tool that adjusts specifically to the patient’s anatomy for high first-pass success,” said Eitan Havis, president, International at Rapid. “It has been a much-needed endeavour to introduce patient-specific stroke procedures to Japan and other Asian countries like China, since there is no room for compromise with the brain.”