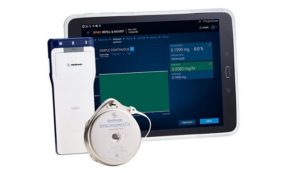
 Medtronic has announced US Food and Drug Administration (FDA) approval of the new SynchroMed II myPTM Personal Therapy Manager for patients with chronic pain. This device enables patients to alleviate their unpredictable pain by delivering on-demand boluses, or drug doses, within therapeutic limits set by their physician.
Medtronic has announced US Food and Drug Administration (FDA) approval of the new SynchroMed II myPTM Personal Therapy Manager for patients with chronic pain. This device enables patients to alleviate their unpredictable pain by delivering on-demand boluses, or drug doses, within therapeutic limits set by their physician.
myPTM works with the SynchroMed II Intrathecal Drug Delivery system, also known as a Medtronic pain pump, which delivers medication directly to the fluid around the spinal cord to relieve chronic pain in appropriate patients. The Medtronic pain pump, an implantable drug pump, provides long-term pain relief at lower doses and with fewer side effects compared to oral pain medications and may allow some patients to eliminate the use of systemic opioids. Personalisation of the therapy gives patients the ability to alleviate unpredictable pain and may further reduce the need for oral opioids.
“Enabling patients to adjust their treatment provides them with some independence to control their pain and gives me confidence knowing that they are getting pain relief without oral opioids. The Medtronic pain pump and myPTM are powerful tools to safely treat chronic pain including intractable cancer pain,” said John A Hatheway, owner and provider at Northwest Pain Care in Spokane, USA.
myPTM is an easy-to-use application on a touchscreen Samsung J3 smart device that is customised to help patients to manage their pain. Healthcare providers can set daily therapeutic doses and allow for on-demand bolusing, or drug delivery, within pre-established limits. myPTM features clear bolus delivery, access to therapy details, and lockout alerts if patient demand exceeds prescribed limits. Physicians also have access to reports that provide insights needed to track progress and collaborate on therapy goals with their patients.
“Samsung and Medtronic have partnered to offer an innovative solution for patients with chronic pain,” said David Rhew, chief medical officer, vice president and general manager for Enterprise Healthcare, Samsung Electronics America. “The ability to directly manage one’s medical condition from a smartphone device is ground breaking and changes the way we think about the personalisation of care.”
“We are striving to simplify targeted drug delivery therapy to make it more accessible. The Control Workflow and Clinician Programmer provide physicians with tools to effectively administer the therapy, and the myPTM provides customised pain relief options for patients,” said Charlie Covert, vice president and general manager of the Targeted Drug Delivery business, part of the Restorative Therapies Group at Medtronic. “As the opioid crisis continues, we are inspired by Medtronic’s mission to continue to innovate and expand access to care for patients who may benefit from our therapies, which have the potential to eliminate the need for oral opioids.”













