Neurolief has announced positive results from a planned interim analysis of the ongoing, randomised controlled MOOD pivotal trial, which is studying Relivion DP—a novel neuromodulation therapy for the treatment of major depressive disorder (MDD).
At a meeting of the study’s independent data monitoring committee (DMC), statistical results from a pre-planned efficacy and safety interim analysis were reviewed. Based on data from 80% of the originally planned study sample size, the DMC issued a recommendation to continue patient enrolment up to the final planned sample size, as the interim results are “positively favourable”.
Full study results will be announced and submitted to the US Food and Drug Administration (FDA), and for CE-mark approval, after the last patient completes the treatment protocol. This is expected in the second quarter of 2024, a Neurolief press release states.
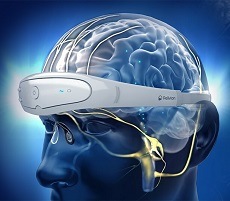
The Relivion DP is a neuromodulation system designed for treatment of depression and has been awarded the US FDA’s Breakthrough Device designation for its novel technology. Similarly to a headset, the patient places the device on their head to administer treatments.
Utilising three adaptive output channels, the device transfers mild electrical pulses to the brainstem via six branches of the occipital and trigeminal nerves. This mechanism stimulates the release of neurotransmitters in the brainstem and modulates brain networks associated with mood.
As part of a digital therapeutics platform, Relivion DP uses a dedicated smartphone application and a cloud database to allow psychiatrists to remotely monitor patients, analyse their data, and personalise treatments to enhance outcomes.
The MOOD trial—conducted in 12 clinical sites across the USA and one in Israel—is a prospective, multicentre, placebo-controlled, randomised, double-blind clinical trial. The study’s primary endpoint assesses changes in depression symptoms from baseline to eight weeks post-treatment initiation, comparing use of Relivion DP to a control group, in patients suffering from MDD who have failed to achieve satisfactory improvement from previous antidepressant medications.
“The successful results of this planned interim analysis are an extremely important milestone for our company,” said Scott Drees, CEO of Neurolief. “MDD is a debilitating condition of epidemic proportion with severe negative impact on patients and humanity. Making this new, promising therapy available is a top priority for Neurolief. It has the potential to be life-changing for treatment-resistant depression patients whose symptoms do not sufficiently improve with antidepressant medications. We can now progress toward making Relivion DP available to the millions of patients in desperate need.”












