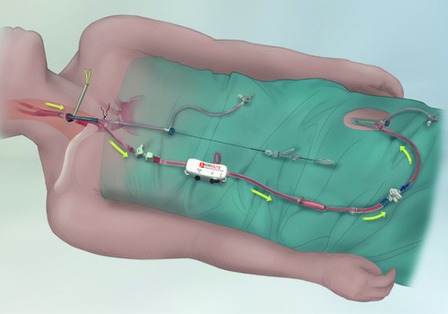
Silk Road Medical has announced that the company has received premarket approval (PMA) from the US Food & Drug Administration (FDA) for the Enroute transcarotid stent system. The Enroute transcarotid stent is the first carotid stent that is introduced and implanted into the carotid artery through a direct common carotid access point to enable a safe and more direct approach to carotid artery stenting.
The Enroute transcarotid stent is indicated for use in high surgical risk patients and is intended to be used in conjunction with Silk Road Medical’s Enroute transcarotid neuroprotection system (NPS), which recently received 510(k) clearance by the FDA. Together the Enroute transcarotid NPS and stent system enables a novel hybrid procedure called transcarotid artery revascularisation (TCAR), which combines surgical principles of neuroprotection with a less invasive stenting procedure. The Enroute transcarotid NPS is a first in class system used to directly access the common carotid artery and initiate high rate temporary blood flow reversal to protect the brain from stroke while delivering and implanting the Enroute transcarotid stent.
The Enroute transcarotid stent was developed pursuant to a license with Cordis Corporation and leverages the micromesh design and long term durability of the Cordis PRECISE carotid stent that was clinically proven in tens of thousands of patients across multiple clinical trials including SAPPHIRE, CASES-PMS and SAPPHIRE Worldwide. The Enroute transcarotid stent has a shorter delivery system optimised for transcarotid access and was recently trialed by leading European physicians.
“TCAR allows us to avoid potential stroke hazards at the aortic arch while placing a stent under robust flow reversal which simulates the superb neuroprotection of CEA,” commented Ralf Kolvenbach, chief of Vascular Surgery at Augusta Hospital, Dusseldorf Catholic Hospital Group. “With the Enroute transcarotid stent we now have a dedicated, ergonomic stent platform for TCAR that combines the control afforded by transcarotid access with the stent’s visibility under X-ray, allowing for confident, precise stent placement.”
The US FDA PMA was based in part on data collected from a subset (52) of 141 High Surgical Risk patients in the ROADSTER study who were treated with the Cordis PRECISE PRO RX stent system and the Enroute transcarotid NPS. Technical success was 100% (52/52) and the Major Adverse Event (MAE) rate at 30 days was 1.9% consisting of a single minor stroke, comparable to the overall ROADSTER results of 3.5% MAE and 1.4% stroke.













