Following an interim analysis of data from the first 182 patients enrolled in DEFUSE 3, the trial has been terminated and is no longer actively enrolling patients. The interim analysis showed a high likelihood of benefit in the endovascular group of the study.
DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3) is a prospective randomised phase III multicentre controlled trial of patients with acute ischaemic anterior circulation strokes due to large artery occlusion treated between six and 16 hours of stroke onset with endovascular thrombectomy therapy plus standard medical therapy versus standard medical therapy. The purpose of DEFUSE 3 is to assess the safety and efficacy of thrombectomy in carefully selected patients in this extended time window. Gregory Albers (Stanford University, Stanford, USA) is the principal investigator of the trial which was conducted by the NIH StrokeNet funded by the National Institute of Neurological Disorders and Stroke (NINDS).
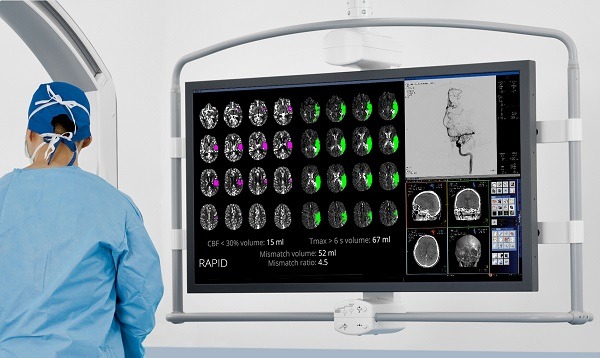
In the study, patients who met the inclusion criteria underwent either CT perfusion/CT angiography (CTP/CTA) or magnetic resonance (MR) diffusion weighted imaging/perfusion weighted imaging/angiography (DWI/PWI/MRA) studies prior to randomisation. These images were processed with an automated image analysis platform (RAPID, iSchemaView) to identify patients with salvageable brain tissue (Target Mismatch Profile). Patients who had evidence of an internal carotid artery (ICA) or middle cerebral artery (MCA) M1 occlusion and a Target Mismatch Profile were randomised in a 1:1 ratio to treatment with one or more FDA-approved thrombectomy devices plus standard medical therapy versus standard medical therapy alone. Selection of the specific device (or devices) was determined by the individual endovascular therapist.
The devices listed in the protocol were: the Trevo Retriever (Stryker), the Solitaire FR Revascularization Device (Medtronic), the Penumbra thrombectomy system (Penumbra) and the Covidien MindFrame Capture Revascularization Device (Medtronic).
The primary endpoint is modified Rankin Score (mRS) at 90 days.
The study planned to randomise up to 476 patients over four years, and it employed a novel adaptive design to identify, at interim analyses, the group with the best prospect for showing benefit from endovascular treatment, based on baseline core lesion volumes and the times since stroke onset.
 The first interim analysis was scheduled to be conducted at 200 patients and the next at 340 patients. But, as announced by Joseph Broderick (University of Cincinnati Gardner Neuroscience Institute, Cincinnati, USA)—who is the principal investigator of NIH StrokeNet National Coordination Center, which represents the infrastructure for DEFUSE 3 and helped to manage the trial—at the Society of NeuroInterventional Surgery 14th Annual Meeting (SNIS; 24–27 July, Colorado Springs, USA), the presentation of the positive DAWN trial in May prompted the first interim analysis of DEFUSE 3 after 182 patients. After review of the available DEFUSE 3 data by the Data Safety and Monitoring Board, DEFUSE 3 was terminated in July due to the high likelihood of benefit in the mechanical thrombectomy group. The investigators, who remain blinded to the study results, will analyse the data after the database is finalised this fall. The trial results are expected to be presented in early 2018 at the International Stroke Conference (ISC; 24–26 January, Los Angeles, USA).
The first interim analysis was scheduled to be conducted at 200 patients and the next at 340 patients. But, as announced by Joseph Broderick (University of Cincinnati Gardner Neuroscience Institute, Cincinnati, USA)—who is the principal investigator of NIH StrokeNet National Coordination Center, which represents the infrastructure for DEFUSE 3 and helped to manage the trial—at the Society of NeuroInterventional Surgery 14th Annual Meeting (SNIS; 24–27 July, Colorado Springs, USA), the presentation of the positive DAWN trial in May prompted the first interim analysis of DEFUSE 3 after 182 patients. After review of the available DEFUSE 3 data by the Data Safety and Monitoring Board, DEFUSE 3 was terminated in July due to the high likelihood of benefit in the mechanical thrombectomy group. The investigators, who remain blinded to the study results, will analyse the data after the database is finalised this fall. The trial results are expected to be presented in early 2018 at the International Stroke Conference (ISC; 24–26 January, Los Angeles, USA).
DEFUSE 3 is not the first trial of its kind to be terminated at the interim analysis due to the high likelihood of benefit in one arm. This has been the trend over the last few years since the MR CLEAN trial, which was investigating the treatment of large vessel occlusion stroke using mechanical thrombectomy versus best medical management, announced positive results in October 2014. The results of MR CLEAN triggered an early evaluation of trial data in a number of similar stroke trials, including ESCAPE, EXTEND-IA, SWIFT PRIME and REVASCAT. The results of these trials combined helped to solidify mechanical thrombectomy as a new standard of care for the treatment of an acute ischaemic stroke with large artery occlusion up to six hours after stroke onset.
 More recently, the DAWN trial, which used the same automated perfusion software (RAPID) as DEFUSE 3 to select patients who were likely to have salvageable tissue in an extended time window, was terminated at its interim analysis and later declared positive. DAWN enrolled selected patients with a symptom onset between six and 24 hours since the patient was last known well and focused on patients with small ischaemic core lesions, where DEFUSE 3 allowed patients with larger core lesions up to 16 hours after symptom onset as long as there was imaging evidence of salvageable tissue. DEFUSE 3 also enrolled patients with milder stroke symptoms (NIHSS ≥6) whereas DAWN required an NIHSS ≥10 and had more complicated entry criteria that combined variations of age, core size, and NIHSS. Because of these differences in selection criteria, DEFUSE 3 was able to enrol a broader patient population (about 40% of the DEFUSE 3 patients would not meet the DAWN selection criteria).
More recently, the DAWN trial, which used the same automated perfusion software (RAPID) as DEFUSE 3 to select patients who were likely to have salvageable tissue in an extended time window, was terminated at its interim analysis and later declared positive. DAWN enrolled selected patients with a symptom onset between six and 24 hours since the patient was last known well and focused on patients with small ischaemic core lesions, where DEFUSE 3 allowed patients with larger core lesions up to 16 hours after symptom onset as long as there was imaging evidence of salvageable tissue. DEFUSE 3 also enrolled patients with milder stroke symptoms (NIHSS ≥6) whereas DAWN required an NIHSS ≥10 and had more complicated entry criteria that combined variations of age, core size, and NIHSS. Because of these differences in selection criteria, DEFUSE 3 was able to enrol a broader patient population (about 40% of the DEFUSE 3 patients would not meet the DAWN selection criteria).
In the case of DAWN, after the first interim analysis allowing trial termination based on efficacy, with 206 patients enrolled, the data and safety monitoring board (DSMB) recommended enrolment be terminated. One hundred and seven patients were randomised to receive the Trevo Retriever and 99 patients to receive medical management.
In terms of time-last-seen-well to randomisation in DAWN, the mean time was 13.4±4.1 (median, 12.2) hours in the treatment arm and 13.0±4.5 (median, 13.2) hours in the control arm (p=0.53), suggesting that more that 50% of the patients were treated beyond 12 hours from time-last-seen-well.
In the treatment arm, procedure duration was 56 minutes median and the total number of Trevo device passes was a median of two. Core lab adjudicated TICIs post-procedure were as follows: modified TICI ≥2b=84%; original TICI ≥2b=72.6%; and TICI 3=10.4%.
In the weighted mRS based co-primary outcome, the mean mRS value in the treatment group was 5.5 versus 3.4 in the control group. The co-primary endpoint of 90-day functional independence was 48.6% in the treatment group versus 13.1% in the control group; a 35.5% actual difference, which is highly significant with a Bayesian probability of superiority of >0.9999. This translates to a number needed to treat of 2.8 to achieve functional independence.
As it relates specifically to the treatment effect in the extended time window in the DAWN trial, treatment effect persisted throughout 24 hours from time-last-seen-well, however, patients treated earlier had better outcomes. “This means that the mantra ‘time is brain’ is unchanged. Eligible patients should still be treated as quickly as possible. It is just that eligibility for treatment should not be restricted by time windows,” principal investigator Tudor Jovin clarified at the time the DAWN results were first announced. The study clearly showed that thrombectomy in patients presenting beyond six hours of time-last-seen-well had a comparable safety profile to thrombectomy performed within six hours.

Only time will tell how the results of DEFUSE 3 will compare to DAWN and how both of these trials will ultimately impact clinical practice. However, as DEFUSE 3 principal investigator Albers told NeuroNews, “Two positive late window trials should have a major impact on how ischaemic stroke patients who present between six and 24 hours are assessed and treated. As both trials relied on perfusion imaging to identify patients, it is likely that perfusion imaging will become the standard for imaging late-window stroke patients. If the substantial efficacy seen in DAWN is confirmed in DEFUSE 3, then it appears that late-window therapy will have a major impact on reducing stroke morbidity.”













Very exciting news !