Tag: RAPID
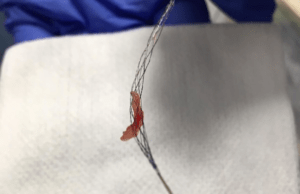
Rapid Medical’s Tigertriever XL receives CE mark approval
Rapid Medical has announced CE mark approval for Tigertriever XL stentriever. The company noted that the first patients have been treated successfully with the...
Rapid ASPECTS receives CADx clearance from the FDA
RapidAI has announced that Rapid ASPECTS has received US Food and Drug Administration (FDA) clearance as the first neuroimaging analysis device in the CADx (computer-assisted...
RAPID platform expands to address haemorrhagic stroke
iSchemaView has launched the newest addition to the RAPID platform, RAPID ICH at the 2019 Congress of Neurological Surgeons (CNS) Annual Meeting (19–23 October,...
RAPID imaging platform expands options for speedy stroke treatment at Atlantic...
Recognising how vital it is that stroke neurosurgeons and neurologists receive timely information about the type of stroke a person is having so they...
iSchemaView’s RAPID approved for use in Brazil
iSchemaView receives approval from ANVISA (the Brazilian Health Regulatory Agency), for the use of the RAPID imaging platform across Brazil.
RAPID is designed to...
iSchemaView and NeuroLogica team up to mobilise stroke care worldwide
iSchemaView and NeuroLogica have announced a partnership to integrate iSchemaView’s RAPID cerebrovascular imaging platform with NeuroLogica’s portable CereTom computed tomography (CT) scanners for use...
iSchemaView completes MDSAP audit — moves to expand worldwide adoption of...
iSchemaView has announced that the company has completed the Medical Device Single Audit Program (MDSAP), with coming certification. iSchemaView is now applying for clearances...
State-of-the-art imaging solution provides automated scoring to assess early signs of...
At the European Stroke Organisation Conference, iSchemaView publicly launched RAPID ASPECTS, a digital imaging solution that assists clinicians in assessing early signs of brain...
RAPID CTA gets FDA clearance
iSchemaView has received final clearance from the US Food and Drug Administration (FDA) for RAPID CTA, the company’s 3D imaging solution for Computed Tomography...
DEFUSE 3 terminated early with high likelihood of benefit in the...
Following an interim analysis of data from the first 182 patients enrolled in DEFUSE 3, the trial has been terminated and is no longer...