Giving blood thinners in addition to clot-busting medications does not improve ischaemic stroke patients’ outcomes at 90 days, according to findings from the MOST trial, which were revealed today in a late-breaking science presentation at the International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
“When we began the trial, we believed the medications would improve outcomes, so we were surprised with the negative results,” said Opeolu Adeoye (Washington University School of Medicine, St Louis, USA), lead author of the study. “However, we designed the trial to allow us to efficiently answer the question for two blood-thinning medications in one trial. We have definitely done that and are pleased with the ability to answer this question.”
The MOST trial—conducted at 57 US centres—was halted in July 2023 after an independent data and safety monitoring board (DSMB) analysed results on the first 500 patients out of a planned 1,200 participants, and determined it “highly unlikely” that a benefit would be found if the research was completed.
The study enrolled adult patients whose ischaemic strokes were severe enough that rehabilitation would likely be needed (National Institutes of Health stroke severity [NIHSS] score ≥6), all of whom received a standard clot-busting medication to dissolve their blood clot—intravenous thrombolysis (IVT)—within three hours of symptom onset or time last seen well.
Participants were then randomised to one of three groups for additional treatment. Some 59 patients received the blood thinner argatroban within 75 minutes of IVT, followed by a 12-hour infusion of argatroban; 228 patients received an initial dose of the blood thinner eptifibatide within 75 minutes of IVT, followed by a two-hour infusion of eptifibatide and a 10-hour infusion of saline placebo; and, in the control group, 227 patients were given IVT and a placebo treatment (12-hour infusion of intravenous saline solution containing neither of the blood-thinning medications). Across all three groups, some 44% of patients also underwent a mechanical thrombectomy procedure.
“A lot of our approaches in stroke treatment were learned from how we treat heart attacks,” Adeoye noted. “In previous trials, we first tested to make sure these medications were safe for use in stroke, and then launched MOST to confirm their safety, and test whether they would work to improve functional outcomes and reduce disability after stroke.”
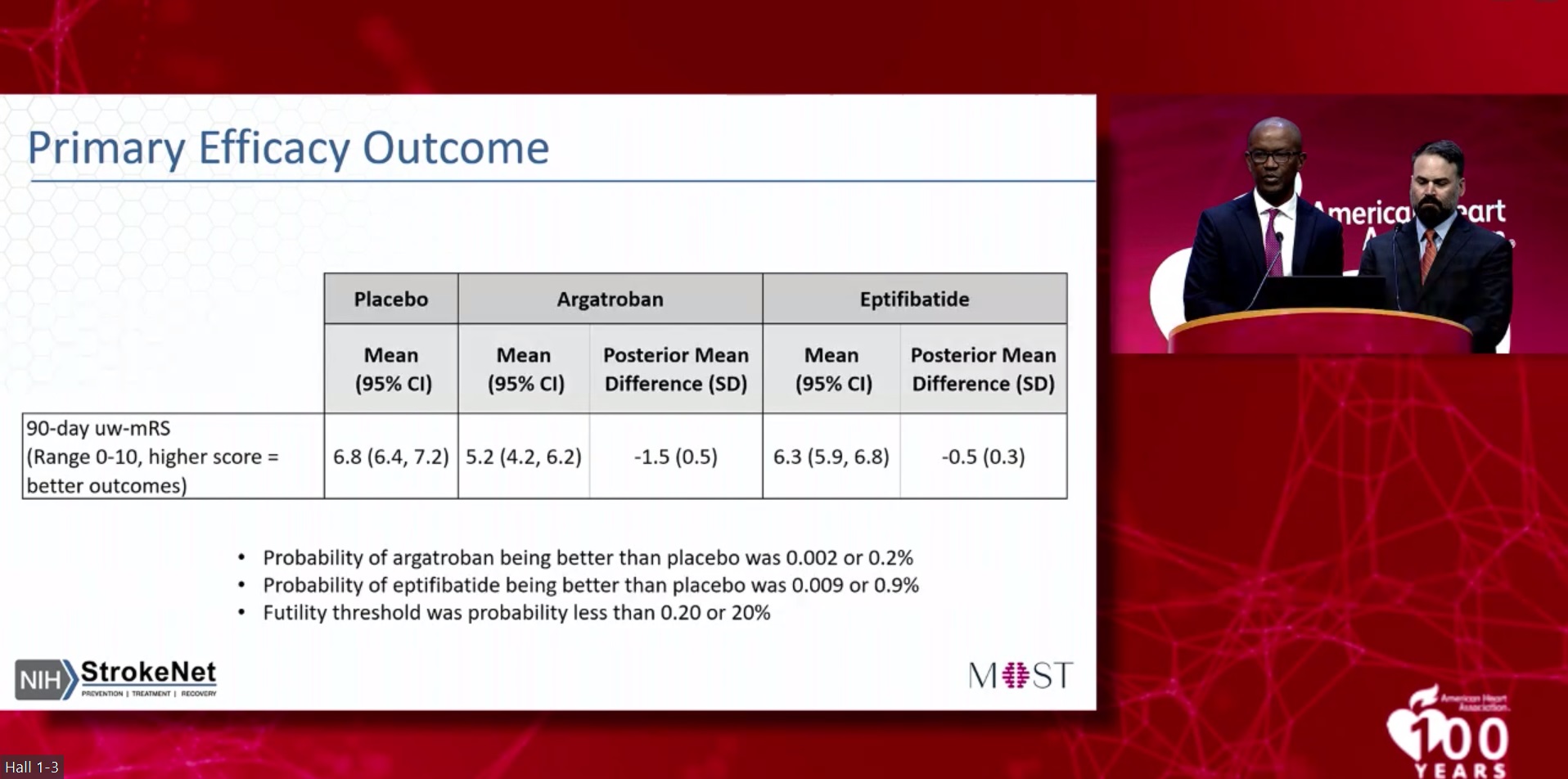
The primary outcome in MOST was the level of physical function at 90 days after ischaemic stroke, assessed using the modified Rankin scale (mRS). MRS scores were translated into a utility-weighted mRS score using validated ratings of functional outcomes by patients and physicians, resulting in a scale of 0–10 on which a higher score indicated a greater treatment benefit. The study’s primary safety measure was the occurrence of symptomatic bleeding in the brain, known as intracranial haemorrhage (ICH), within 36 hours of receiving one of the two blood thinners, with this outcome being reviewed by the DSMB after every 30 patients enrolled.
Medical professionals providing care in the trial were aware of whether a blood thinner or placebo had been given to each patient, but neither the patients nor the clinicians rating their outcomes—independent neurologist reviewers using videotaped assessments—were aware of which treatment patients had received.
The DSMB’s interim analysis—which was planned from the start of the study—ultimately included data from 514 enrolled patients (average age, 68 years; 50% women), and found that the two blood-thinning medications used did not significantly increase the risk of ICH. However, neither drug improved functional outcomes in the trial either. Patients in the control group, who received IVT plus placebo, had an average utility-weighted mRS score of 6.8, compared to 5.2 in those who received argatroban and 6.3 with eptifibatide.
“In addition, we were not able to address the possible benefit of giving these or similar blood thinners directly into an artery in the area of the stroke, rather than giving the medications systemically through a vein, as done in this trial,” Adeoye concluded.
On this front, multiple studies involving patients receiving a thrombectomy to remove their stroke-causing clots, intended to determine whether delivering blood thinners into the affected artery may improve outcomes, are currently underway.