Ten years ago, mechanical thrombectomy demonstrated safety and superior efficacy to the prior standard of care in acute ischaemic stroke across a series of randomised clinical trials, and very few people would argue that the field has witnessed a comparably significant advance in the decade since. However, if the positive signals being reported by innovative startup Quantanosis continue to deliver on their early promise, Ameer Hassan (Valley Baptist Medical System, Harlingen, USA)—the company’s co-founder and chief executive officer (CEO)—believes the stroke field could be about to witness its most groundbreaking development for quite some time.
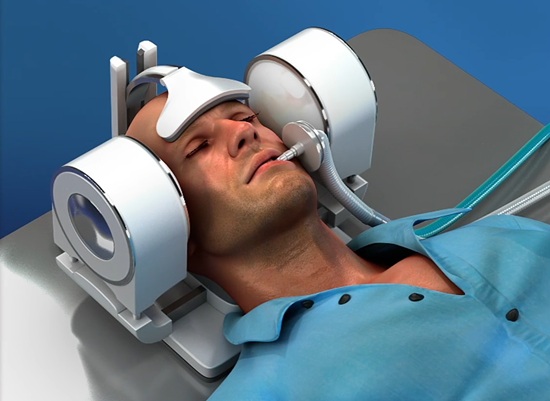
In a nutshell, Quantanosis’ technology is a portable, non-invasive, robotic platform for diagnosing and treating acute ischaemic strokes via a combination of transcranial Doppler (TCD) ultrasound, artificial intelligence (AI) and histotripsy. By introducing a system that is operator-independent while also creating a therapeutic option that overcomes many of the issues faced by thrombectomy treatments and thrombolytic drugs, its overall goal is to “democratise” stroke care.
The system is akin to a headset, through which TCD ultrasound imaging is initially administered. And, upon AI analyses of this diagnostic scan revealing a large vessel occlusion (LVO) in the M1 or proximal M2 segment of the patient’s middle cerebral artery (MCA)—or in their carotid terminus—rapid bursts of focused ultrasound energy are delivered via a spherical annular array. These ultrasound bursts target the middle of the stroke-causing clot to break it up, homing in on an area that is less than half the typical size of the MCA at 2.7mm by 1.3mm. Two ultrasound probes hit the proximal and distal ends of the clot simultaneously—a double-pronged attack that erodes and ultimately dismantles the clot via shock-scattering histotripsy. Or, as Hassan more trendily describes it, “a tornado of bubbles that the clot cannot pass without getting destroyed”.
“The way I like to explain it is that it’s like lightly slapping the clot a thousand times per second,” Hassan says. “You’re trying to shear off pieces that are so small in size—our original goal was 250µ, but now we’re getting to 90% of clot remnants being less than 100µ. Think of this as mechanical thrombolysis with an acoustic scalpel. We can target the clot with precision of less than 0.5mm, with no catheters, no wires and no lytic drugs.”
On this point, Hassan notes that—if it gains regulatory approval, and can be made available at every urgent, primary and comprehensive care centre across the USA—one of the real-world advantages offered by Quantanosis’ technology is “truly expanding” timely access to stroke treatments.
“Thrombolytics can only be given up to 4.5 hours [from symptom onset],” he explains. “And, there are around 260 comprehensive stroke centres and 2,500 primary stroke centres in the USA, and the average difference between mechanical thrombectomy treatment times for an M1 occlusion at a comprehensive stroke centre versus being transferred from a primary stroke centre is almost two hours. So, if we can treat patients two hours earlier—and we save two million neurons every minute—that’s 240 million neurons we’re saving by getting a patient recanalised at a primary centre before shipping them to a comprehensive centre if they need any further services.”
Hassan does acknowledge that the Quantanosis platform will not be the perfect solution in every LVO stroke; patients with thicker skulls or very calcified clots will be less amenable to the histotripsy treatment, and are therefore likely to require a thrombectomy procedure within an actual cath lab. However, these patients account for a fraction of the LVO stroke population, with Hassan anticipating that Quantanosis’ technology will be able to successfully treat close to 70% of all M1 occlusions.
“And, in 10 years’ time, because this is so easy—you’re pushing one button to initiate the TCD scan and one button to initiate the histotripsy if there’s an LVO—this will be going directly to a person with suspected stroke via ambulance,” he continues. “In my mind, it’ll be in shopping malls, event centres and airports, almost like an AED [automated external defibrillator] for stroke. That’s the way we envision it.”
Quantanosis’ origin story
During his tenure as president-elect of the Society of Vascular and Interventional Neurology (SVIN), one of Hassan’s key areas of focus was Mission Thrombectomy (MT)—an initiative intended to improve access to timely stroke care across the globe. Through this, he was supporting Wondwossen Tekle’s (University of Texas Rio Grande Valley, Edinburg, USA) attempts to establish the first comprehensive stroke centre in Ethiopia.
While these efforts were ultimately successful, with the country’s first comprehensive stroke centre being inaugurated in 2022, it was reliant on financial contributions from industry partners and required Tekle himself to commit a significant amount of time to unpaid clinical work. Hassan notes that the endeavour put into perspective how much of an uphill battle it will be to improve the 2.7% rate of global access to thrombectomy within two hours of symptom onset observed in the MT-GLASS study.

“I can’t find 50–100 martyrs like Wondwossen, who’s willing to give up most of his salary and go to his [home] country to work for free for five years—he’s basically donating all of his time,” Hassan explains. “So, I started to think about how we can do this in a way where it’s done consistently all over the world.”
This conundrum led Hassan to begin ideating approaches involving reusable devices, initially through a reusable histotripsy catheter that could be sterilised between procedures, thus circumventing the significant costs associated with current stent retrievers and aspiration catheters. But, having run into “a lot of roadblocks”—chiefly, the fact that ultrasound catheters tend to be too rigid to reach the MCA’s M1 segment—he recalls that the “eureka moment” came when one of his colleagues suggested administering ultrasound from outside of the blood vessel as opposed to endovascularly.
“We came up with the idea, we worked with a couple of engineers, we started using a simulator and we tested it,” Hassan says. “Then, we needed to find a company that could build it. I called six American companies and all of them told me, ‘you’re crazy, this is impossible to build’. Then, [Quantanosis co-founder] Yousef Khalili made some phone calls in Germany and he found a company that makes ultrasound equipment for removing oil from oil rigs. I thought that was cool, but it’s not the reason we worked with them; they have an amazing R&D [research and development] department, so they can fabricate anything you want—and their head of R&D did his PhD in histotripsy! And, when he saw this project, he said he could build what we needed and, as they say, the rest is history.”
Recent milestones
Initial prototypes of the system were developed and tested as early as 2022, and demonstrated an ability to break up blood clots within two minutes. According to Hassan, he and the team spent the subsequent two-and-a-half years working to make the technology more accurate, more consistent, and faster, finetuning its ultrasound capabilities and updating its geometric design to incorporate “multiple probes within a probe” in order to offer both diagnostic and therapeutic capabilities.
A recent conversation with Adnan Siddiqui (University at Buffalo, Buffalo, USA)—who told Hassan that the US Food and Drug Administration (FDA) would “never accept” a regulatory submission backed solely by perfused cadaver studies—led the Quantanosis team to begin testing its technology in live-tissue models at Siddiqui’s New York-based Jacobs Institute. This research produced positive findings, as the system successfully broke up clot in a porcine carotid artery without causing any damage to the neurovasculature.
Following a subsequent request from the US FDA, the company is now carrying out further animal-model testing at the Jacobs Institute, in addition to raising funds through a Series A financing round and attempting to bring in multiple business leaders including a new president, chief operating officer (COO) and chief engineer. According to Hassan, Quantanosis will soon look to gain US FDA Breakthrough Device designation for its system as well as investigational device exemption (IDE) approval to conduct a first-in-human study involving 10 patients at Valley Baptist Medical Center, with the latter potentially getting underway in the second half of 2026.
“Every [first-in-human] patient would get CTA [computed tomography angiography] perfusion imaging, these would be non-tPA [tissue plasminogen activator] candidates— most likely due to time constraints or anticoagulant medications—where we know they have an M1 occlusion,” he elaborates. “We intubate the patient, target and hit the clot, and then every patient would go straight to the cath lab to confirm recanalisation with angiography. And if we can get TICI [thrombolysis in cerebral infarction] 2b–3 in at least 50% of these patients, we’ve revolutionised stroke care.”
Hassan’s belief is that this technology could “open the door” to interventional treatment, not only for thrombectomy-eligible patients at primary stroke centres, but also the wide array of patients who have traditionally been ineligible for intravenous thrombolytics—a significant achievement given that just 8% of US stroke patients currently receive tPA.
“If we can double or triple that, it would be huge,” he adds. “We’re hoping to shift patients’ stroke treatment forward by about 90 minutes, and I believe we’ll get there.”
One of the more recent major steps Quantanosis has taken towards realising this goal came in the form of a proof-of-concept study published in the journal Interventional Neuroradiology earlier this year. Authored by Hassan, Khalili and Thomas Dreyer—the aforementioned head of R&D at German company Weber Ultrasonics—the study sought to assess the feasibility of using histotripsy to precisely liquefy artificial clot analogues within an experimental setting. According to the paper, focused ultrasound was applied using optimised parameters, including pulses per burst, repetition rates, and output amplitudes, with these parameters being systematically adjusted to determine the most effective settings for clot ablation. A key intention of the researchers was “maximising clot liquefaction while minimising residual fragments”.
“Until now, we’ve never been able to prove that histotripsy breaks up large-vessel thrombi, at least in anything mimicking the size of clots in the MCA,” Hassan avers. “[In the study], we achieved full patency in 45–90 seconds in most of those tests, with complete flow restoration, and all of the clot debris was smaller than 250µ and the majority was smaller than 100µ.”
Following hot on the heels of this paper, Quantanosis recently submitted the findings of its latest animal-model testing for publication, and is set to update the neurointerventional community on its current progress at the 2025 SVIN annual meeting (20–22 November, Orlando, USA).
The future of stroke care
“I do think this will replace thrombolysis for LVO strokes,” Hassan opines. “We believe that about 20% of all strokes are LVOs, and—if we can show superiority when it comes to safety, because we’re not causing the same rate of haemorrhages as tPA—I don’t think those patients will receive thrombolytics anymore, because people would rather get an ultrasound than have thrombolytics circulating their whole body.”
Here, Hassan also cites the low recanalisation rates that tPA and tenecteplase (TNK) are able to achieve in M1 occlusions (15–20%), and carotid occlusions (6%), as another context within which Quantanosis’ technology—even by attaining recanalisation in around 50% of cases—may be able to improve on the outcomes associated with the current standard of care.
The second key area where Hassan anticipates the technology playing a major role is in patients presenting beyond the thrombolytic time window of 4.5 hours, and those on anticoagulation therapy, who are ineligible to receive tPA—a “huge part” of the ischaemic stroke population.
“In my mind, [this will] be in shopping malls, event centres and airports, almost like an AED for stroke. That’s the way we envision it.”
“But, right now, I think we’d be taking the biggest chunk [of patients] away from mechanical thrombectomy,” he says. “We’d be taking the carotid T, M1 and proximal M2 business, which is the majority today. It’s probably 75% of mechanical thrombectomy treatments. We’d leave D/MeVOs [distal/medium-vessel occlusions], distal branches and basilar artery occlusions to thrombectomy, because our technology is built for the temporal window, which involves a straight shot at the carotid T and MCA. Could it be reconfigured for other vessels? Probably, but that’s likely to be about 10 years down the road. Right now, we want to get this up and running quickly, and target the big fish: the 70% of LVO patients who need it today.”
Mapping out how the technology is likely to be integrated into care systems over the next decade, Hassan says that he hopes it will subsequently expand into urgent care facilities, primary stroke centres, and—eventually—emergency medical services (EMS), before perhaps one day even replacing thrombectomy procedures entirely.
The advent of mechanical thrombectomy is widely considered to be nothing short of a miracle in the treatment of acute ischaemic stroke. It has frequently been cited as the single most impactful breakthrough in the recent history of this space—including by Hassan himself, who, speaking to NeuroNews in 2023, said the procedure was “hands down” the neurological field’s most important achievement.
“In the last three decades, no other treatment has exhibited such a remarkably low number needed to treat, nor facilitated the recovery of [so many] individuals affected by a life-threatening and debilitating disease,” he asserted.
However, no medical marvel is without its limitations, and thrombectomy’s biggest pitfall—perhaps second only to low rates of global access—is the relatively high occurrence of intracranial haemorrhage (ICH) associated with the procedure. Despite the phenomenon of reperfusion injury meaning that bleeding-related complications are essentially inevitable in some cases, Hassan believes that, by achieving sufficient recanalisation while preventing the significant portion of the ICHs seen following thrombectomy that are attributable to the procedure itself, Quantanosis’ technology will able to successfully navigate the first-in-human and pivotal studies it is planning over the next few years.
Forecasting how utilisation of Quantanosis’ system might look in the real world, Hassan anticipates it not only providing a faster and safer alternative to the current standard of care, but also a more cost-efficient one.
“High-volume centres,” he suggests, “will basically get an annual, ‘all-you-can-treat’ subscription, while smaller institutions—like urgent care and primary stroke centres—will have to pay an annual lease where they get charged every time they press that button. Those fees would be significantly less than the cost of thrombolytic drugs today, and definitely a lot less than the cost of thrombectomy devices.”
Finally, returning to the problem that initially sparked the idea for Quantanosis, Hassan details his company’s intention to “heavily subsidise” usage of the system in Ethiopia, Egypt, rural parts of India and the many other regions that are presently unable to afford adequate interventional stroke care.
“The main reason we did this was to find a way of truly improving mechanical thrombectomy access and democratising stroke care, and I would say that’s the take-home message: we’re democratising stroke care, without expensive drugs or devices,” he concludes.









