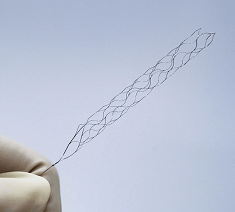
Phenox, a Wallaby Medical company, has announced the US Food and Drug Administration (FDA) 510(k) clearance of its Preset thrombectomy device for the treatment of acute ischaemic stroke.
The Preset product family, which has been available in Europe for more than 10 years, is now cleared for use in the USA.
Preset is a minimally invasive device used to remove blood clots that cause acute ischaemic strokes via a mechanical thrombectomy procedure. It is designed to be easy to use and can be deployed quickly, making it an ideal option for hospitals and clinics treating acute ischaemic stroke patients, according to a Phenox press release.
The device is indicated to reduce disability in patients with a persistent, proximal large vessel occlusion in the anterior circulation, and smaller core infarcts, who have first received thrombolytic therapy. Phenox claims to be only the third company to receive this device indication in the USA.
“We are thrilled to be able to offer our Preset thrombectomy device to physicians and their patients in the USA,” said Andrew Cormack, chief commercial officer at Phenox. “The Preset device has been used successfully in Europe for over a decade, and we are confident that it will be well-received by US physicians as well.
“We believe that this device will play a critical role in the treatment of acute ischaemic stroke, and we expect it to yield significant growth for our company.”












