Tag: essential tremor
Insightec launches next-generation Exablate Prime neurosurgery platform in Europe
Insightec has announced the CE-mark approval and European launch of Exablate Prime, which the company describes as a “significant enhancement” to its Exablate Neuro...
Insightec gains US FDA nod for five-year endpoint in MR-guided focused...
Insightec has announced US Food and Drug Administration (FDA) approval of the five-year study endpoint for what it claims is the largest prospective, long-term...
Insightec receives US FDA approval to treat essential tremor patients’ second...
Insightec recently announced it has received an additional US Food and Drug Administration (FDA) approval for treatment of essential tremor using its Exablate Neuro...
Insightec announces sustained and significant tremor improvement with focused ultrasound thalamotomy
Insightec has announced the publication of what it claims is the largest prospective, long-term follow-up study to date of unilateral magnetic resonance imaging-guided focused...
Insightec announces financing up to US$200 million to support continued growth
Insightec has announced that it has signed a credit facility for up to US$200 million with affiliates of existing shareholders, Perceptive Advisors and Community Fund....
Indian health ministry approves Exablate Neuro focused ultrasound platform
Insightec has announced its incisionless neurosurgery platform, the Exablate 4000, has received market approval by the Central Drugs Standard Control Organization (CDSCO)—part of the Ministry...
Insightec announces Seoul National University Hospital is installing its Exablate Neuro...
Insightec has announced that Seoul National University Hospital (SNUH) is installing the company’s Exablate Neuro (Exablate 4000) platform—which uses focused ultrasound to ablate targets...
Insightec announces approval of Exablate Neuro focused ultrasound platform in Singapore
Insightec has announced approval of its Exablate Neuro platform by the Health Sciences Authority (HSA) in Singapore. The Exablate Neuro (Exablate 4000) platform uses...
Abbott receives CE mark of MR-conditional labelling for Infinity deep brain...
Parkinson’s disease, dystonia and essential tremor patients in CE Mark countries will now benefit from upgraded functionality and new magnetic resonance (MR) conditional labelling...
Focused ultrasound earns positive NICE guidance for treatment of essential tremor
INSIGHTEC has announced that The National Institute for Health and Care Excellence (NICE) issued a positive NICE guidance for unilateral MRI-guided focused ultrasound thalamotomy...
The Japanese Ministry of Health, Labour and Welfare approves Exablate Neuro...
The Japanese Ministry of Health, Labour and Welfare (MHLW) has approved InSightec’s Exablate Neuro system for the non-invasive treatment of essential tremor in patients...
Infinity deep brain stimulation system approved in the USA
St Jude Medical has announced the United States Food and Drug Administration approval and first implant of the Infinity deep brain stimulation (DBS) system...
FDA approves first MRI-guided focused ultrasound device to treat essential tremor
The US Food and Drug Administration has approved the first focused ultrasound device to treat essential tremor in patients who have not responded to...
Health Canada approves Insightec’s Exablate Neuro system for the treatment of...
INSIGHTEC has announced that Health Canada has approved its Exablate Neuro system for the treatment of essential tremor.
INSIGHTEC’s Exablate Neuro platform is transforming medicine...
Insightec broadcasts the first live Exablate Neuro brain treatment at ISTU...
At the 16th International Symposium on Therapeutic Ultrasound 2016 (ISTU) in Tel Aviv, Israel, INSIGHTEC hosted a first time live broadcast from the MRI room...
InSightec secures US$22 million and appoints Maurice R Ferré as chief...
InSightec has secured a US$22 million as part of its round D investment. This investment has been led by its current shareholders. In addition,...
Exablate Neuro platform approved by Korean Ministry of Food and Drug...
Elbit Imaging, a shareholder in InSightec, has announced that the company’s Exablate Neuro platform has been approved for the treatment of movement, pain and...
Insightec submits pre-market application for FDA approval of Exablate Neuro for...
Insightec has announced that it has submitted a pre-market approval application (PMA) to the US Food and Drug Administration (FDA) for its Exablate Neuro...
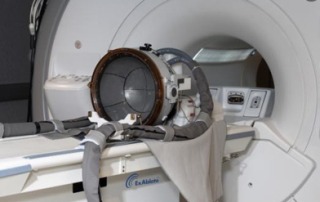
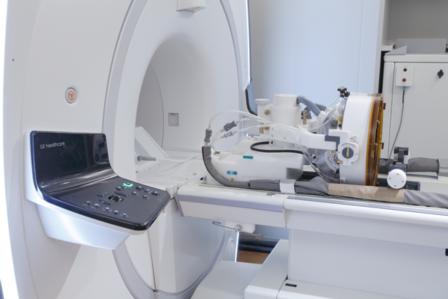
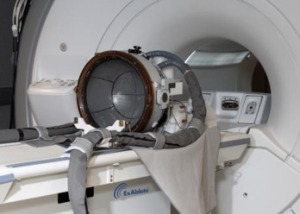
Insightec announces launch of Neurosurgery System on 1.5T MRI platform
Insightec has expanded its non-invasive neurosurgery system from 3T to 1.5T magnetic resonance imaging (MRI) systems. This launch expands the potential market for Insightec’s ExAblate...