Tag: brain monitoring
Zeto obtains US FDA 510(k) clearance for next-generation brain monitoring product
Zeto has announced that its innovative, next-generation brain monitoring product, named the ONE, has received 510(k) clearance from the US Food and Drug Administration (FDA).
"Zeto...
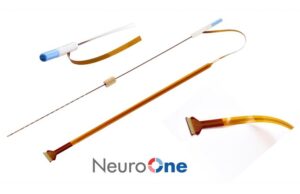
NeuroOne announces US commercial launch of Evo sEEG electrodes
NeuroOne Medical Technologies has announced the commercial launch of the Evo stereoelectroencephalography (sEEG) electrode product line in the USA.
Zimmer Biomet holds exclusive worldwide distribution rights...
NeuroOne receives US FDA 510(k) clearance to market Evo sEEG system...
NeuroOne Medical Technologies has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market its Evo stereoelectroencephalography (sEEG) electrode technology...
NeuroOne submits special 510(k) to US FDA for Evo sEEG electrode
NeuroOne Medical Technologies has announced the submission of a special 510(k) to the US Food and Drug Administration (FDA) for its stereoelectroencephalography (sEEG) electrode...
NeuroOne releases successful long-term recording data with novel electrode technology
NeuroOne Medical Technologies has announced that it successfully completed initial preclinical, long-term testing of recording capabilities on its thin-film platform electrode technology.
In test...
Wyss Center, Inselspital Bern announce clinical trial of long-term brain monitoring...
As part of a recently announced clinical trial, brain signal recording with the Wyss Center for Bio and Neuroengineering’s novel brain-monitoring technology, its subscalp...