Tag: Avicenna.AI
Avicenna.AI secures US FDA 510(k) clearance for two AI-powered imaging tools
Avicenna.AI announced today that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for its CINA-ASPECTS and CINA-iPE products.
CINA-ASPECTS is...
Incepto partners with Avicenna.AI to improve stroke management in Europe
Incepto, a distributor and co-creator of artificial intelligence (AI) solutions applied to medical imaging, has signed a partnership with Avicenna.AI to enrich its platform...
Avicenna.AI announces total funding of US$10 million after securing Series A...
Avicenna.AI announced today that it has secured a Series A funding round, bringing aggregated investment in the company close to US$10 million. The round...
Avicenna.AI partners with CARPL.ai to provide AI-powered radiology solutions
Avicenna.AI has today announced a distribution agreement with CARPL.ai—a company providing a technology platform that enables service-provider-oriented testing, validation and deployment of artificial intelligence...
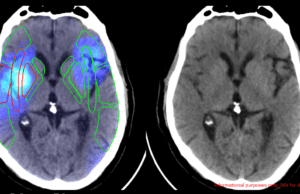
Avicenna.AI signs distribution agreement with Sectra for neurovascular AI solutions
Avicenna.AI has announced a signed distribution agreement with Sectra—an international medical imaging information technology (IT) and cybersecurity company. The agreement will see Avicenna’s AI...
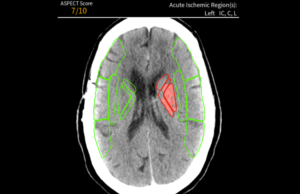
Avicenna.AI receives CE mark for AI tool to assess stroke severity
Medical imaging AI specialist Avicenna.AI has received CE mark certification for its CINA ASPECTS AI tool for stroke severity assessment. CINA ASPECTS automatically processes...
Avicenna.AI Stroke detection tool approved for Medicare NTAP
Aviceanna.AI has announced that its medical imaging artificial intelligence (AI) tool, CINA Head, has qualified for the new technology add-on payment (NTAP) recently approved...
Avicenna AI announces partnership with Canon Medical Systems
Avicenna AI has announced they will partner with Canon Medical Systems to deliver a fully integrated stroke detection artificial intelligence (AI) solution.
Avicenna AI specialises...
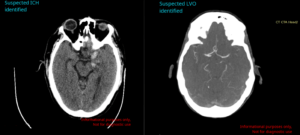
Avicenna.AI secures FDA clearance for its CINA Head neurovascular imaging artificial...
Medical imaging artificial intelligence (AI) specialist Avicenna.AI has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for its...