Tag: aspiration thrombectomy
Imperative enhances Zoom stroke system with 4S catheter launch
Imperative Care has announced the commercial launch of the Zoom 4S catheter—an advanced aspiration thrombectomy device—and the first acute ischaemic stroke patient cases with...
Von Vascular’s SMARTstatic technology outperforms standard static aspiration in benchtop study
Von Vascular has announced the presentation of new data evaluating its Algo smart pump at the 2025 BRAIN conference (1–3 December, London, UK), with...
In-vivo thrombectomy data indicate improved clot ingestion and FPE rates with...
Von Vascular has announced what it describes as “compelling” new in-vivo data on its Algo smart pump featuring adaptive pulsatile aspiration (APA). These data...
More work needed in MeVOs—but cyclic aspiration could be thrombectomy’s next...
Given the procedure’s long-proven effectiveness, present-day advances in stroke thrombectomy treatments tend to be relatively incremental. That said, Jan Gralla (University Hospital of Bern,...
Aspiration thrombectomy produces notably lower sICH and vasospasm rates versus stent...
Analyses of a large US registry comparing first-line stroke thrombectomy approaches for medium-vessel occlusion (MeVO) stroke have shown that direct aspiration is associated with...
Imperative announces new data supporting use of Zoom system in M2...
Imperative Care has today announced late-breaking data from the Imperative trial evaluating aspiration thrombectomy via the Zoom system in stroke patients with M2 occlusions,...
Anaconda Biomed receives CE-mark certification for ANA5 funnel catheter
Anaconda Biomed has today announced that it has received CE-mark certification for its ANA5 funnel catheter. The CE marking confirms that the ANA5 device...
Precise catheter sizing holds key to unlocking ‘maximum benefit’ of aspiration...
This advertorial, intended only for audiences outside the USA, is sponsored by Penumbra.
“Aspiration used to be something straightforward, almost like a ‘no-brainer’, whereas now...
Von Vascular reports superior complete clot ingestion versus leading aspiration pump...
Von Vascular has announced the publication of a pivotal preclinical research study evaluating the performance of its Algo smart pump technology used in ‘smart...
If at first you don’t succeed, switch and try again
This advertorial is sponsored by Medtronic.
Despite the popularity that aspiration-first stroke thrombectomy has gained in recent years, the approach has its limitations—and, on occasions...
Route 92 announces presentation of SUMMIT MAX results and gains new...
Today, Route 92 Medical has announced positive results from its SUMMIT MAX randomised controlled trial (RCT) comparing the efficacy and safety of the HiPoint...
Imperative Care launches dual aspiration thrombectomy catheter for ischaemic stroke treatment
Imperative Care has announced that the Zoom stroke system has been further expanded with the launch of the Zoom DuoPort technology, which allows physicians...
Imperative announces randomised trial assessing aspiration thrombectomy in M2 occlusion strokes
Imperative Care has announced today that it will fund an investigator-initiated, multicentre randomised controlled clinical trial comparing aspiration thrombectomy with the Zoom stroke system...
New technologies and improved understanding ensure aspiration thrombectomy will continue to...
This advertorial, intended only for audiences outside the USA, is sponsored by Penumbra.
Recently, at the 2024 BRAIN conference (2–4 December, London, UK), David Volders...
Imperative secures US FDA 510(k) clearance of Zoom system for stroke...
Imperative Care has today announced the receipt of US Food and Drug Administration (FDA) 510(k) clearance of the company’s Zoom system, making it the first...
Von Vascular presents early data on Algo smart pump at BRAIN...
Von Vascular has announced the first international presentation of data on its Algo smart pump at the 2024 BRAIN conference (2–4 December, London, UK). The...
RapidPulse receives US FDA approval for IDE study of innovative cyclic...
RapidPulse announced today that the US Food and Drug Administration (FDA) has agreed that the company can begin enrolment in an investigational device exemption...
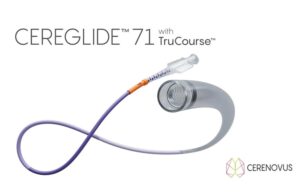
Physician feedback makes CEREGLIDE 71 a new gold-standard contender among stroke...
This advertorial, intended for readers outside the USA only, is sponsored by Johnson & Johnson MedTech Neurovascular.
“If you take all the good things about...
Penumbra announces completion of enrolment in THUNDER IDE study
Penumbra has announced the completion of enrolment in its THUNDER investigational device exemption (IDE) clinical study for patients with acute ischaemic stroke.
THUNDER is evaluating...
Toro Neurovascular announces first patient treatment in clinical trial of SuperBore...
Toro Neurovascular has announced the successful treatment of the first patient using its Toro 88 SuperBore aspiration catheter as part of a first-in-man clinical...
New research highlights novel ‘complete clot ingestion’ metric for aspiration thrombectomy
Potentially groundbreaking research was presented recently at the Society of NeuroInterventional Surgery (SNIS) annual meeting (22–26 July, Colorado Springs, USA), with Robert Starke (University...
Ceretrieve announces successful results in FIH study of stroke aspiration catheter
Ceretrieve has announced the successful results of the company's multicentre, single-arm study, showcasing the capabilities of its “state-of-the-art” aspiration catheter in stroke treatment.
The study—conducted across two...
Hippo-Cheetah combination: a whole new animal in aspiration thrombectomy
This advertorial, intended for US readers only, is sponsored by Q’Apel Medical.
Hot on the heels of the device’s Food and Drug Administration (FDA) clearance...
Penumbra launches Midway intermediate catheters and expands European stroke portfolio
Today, Penumbra announced the launch of its Midway 43 and Midway 62 delivery catheters, which are designed to provide ideal tracking and a stable...
Route 92 announces US FDA 510(k) clearance for FreeClimb 54 reperfusion...
Route 92 Medical has today announced receipt of US Food and Drug Administration (FDA) 510(k) clearance for the FreeClimb 54 reperfusion system, comprised of...
Cerenovus announces European launch of Cereglide 71 aspiration catheter for acute...
Cerenovus, part of Johnson & Johnson MedTech, has today announced the launch of its Cereglide 71 aspiration catheter in Europe, an aspiration catheter equipped...
RapidPulse’s precise cyclic aspiration technology “highly effective and safe” in LVO...
A study recently published online in the journal Interventional Neuroradiology has provided multicentre trial results that demonstrate the promise of using precise cyclic aspiration in the...
Route 92 completes enrolment in 250-patient SUMMIT MAX trial
Route 92 Medical has today announced the completion of enrolment in the 250-patient SUMMIT MAX clinical trial evaluating the safety and effectiveness of its...
Mivi Neuroscience presents primary endpoint results from EvaQ trial
Mivi Neuroscience has announced the primary endpoint results of its EvaQ trial—a prospective, multicentre, global, single-arm, US Food and Drug Administration (FDA)-regulated investigational device...
Cerenovus launches next-generation Cereglide 71 catheter for ischaemic stroke revascularisation
Cerenovus, part of Johnson & Johnson MedTech, has announced the launch of its Cereglide 71 device—a next-generation intermediate catheter with TruCourse technology indicated for...
New SOFAST data show high technical success and first-pass rates with...
Fresh data from the SOFAST study have indicated high rates of technical success and first-pass reperfusion with the Sofia 6Fr Flow Plus aspiration catheter...
New data suggest faster and less costly thrombectomy procedures with Zoom...
Imperative Care recently announced the publication of clinical data in the journal Interventional Neuroradiology (INR) detailing the use of its Zoom aspiration catheters in...
The physics of aspiration thrombectomy—what matters?
This advertorial, intended for readers outside the USA only, is sponsored by Penumbra.
Much of the clinical success that can be achieved with aspiration-based mechanical...
Imperative announces late-breaking data showing benefits of ‘angled-tip’ aspiration catheters
Imperative Care has announced late-breaking data from the Neurovascular Quality Initiative-Quality Outcome Database (NVQI-QOD) evaluating its Zoom stroke solution, which were presented at the...
“No significant difference” between aspiration and stent-retriever thrombectomy in medium vessel...
When utilised as a first-line technique for medium vessel occlusion (MeVO) stroke, aspiration and stent-retriever thrombectomy have demonstrated “no significant difference” in imaging-related or...
Combined thrombectomy technique fails to demonstrate superiority over aspiration alone
New data from the VECTOR randomised controlled trial—presented at the European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany)—have indicated that a mechanical thrombectomy...
Paediatric case helps highlight benefit of ‘aspiration first’ in young stroke...
A unique case presentation at this year’s LINNC Americas Seminar (16–17 March, Miami, USA) saw Rafael de Oliveira Sillero (University of Texas Southwestern Medical...
US physicians publish “first reported use” of Indigo aspiration system for...
Physicians in the USA have published details of—to their knowledge—the “first reported use” of the Indigo aspiration system (Penumbra) to treat cerebral venous sinus...
Route 92 publishes data demonstrating 80% first-pass effect with novel thrombectomy...
Route 92 Medical has announced the publication of initial results from the SUMMIT NZ clinical trial—a single-arm, multicentre, prospective trial evaluating its proprietary Monopoint...
Penumbra receives FDA clearance for RED 62 reperfusion catheter
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance and commercial availability of the RED 62 reperfusion catheter—the latest addition to the...
HeMo Bioengineering receives China’s NMPA approval for Afentta aspiration catheter
HeMo Bioengineering, a Singapore-based medical device company with a focus on treating stroke patients, has announced that its Afentta intracranial thrombectomy aspiration catheter—a product...
Clot permeability linked to first-attempt success of aspiration thrombectomy
A multicentre study has reported that clot perviousness or permeability, the ability for contrast used during the initial imaging workup to seep through a...