Tag: aspiration catheter
New physician preference data on Sofia Flow 88 aspiration catheter revealed...
Terumo Neuro has unveiled new, real-world physician preference data from the Europe, Middle East and Africa (EMEA) region on the Sofia Flow 88 aspiration catheter. The...
J&J highlights neurovascular advances at ESMINT 2025 following CE-mark approval for...
Johnson & Johnson (J&J) MedTech highlighted its latest clinical and technological advancements recently at the European Society of Minimally Invasive Neurological Therapy (ESMINT) annual congress...
Imperative Care secures US FDA clearance for Zoom 7X catheter alongside...
Imperative Care has announced US Food and Drug Administration (FDA) 510(k) clearance for its latest advancement in ischaemic stroke treatment, the Zoom 7X aspiration...
Late-breaking MARRS trial data reveal 61% first-pass effect rate with Perfuze’s...
Perfuze has announced the presentation of positive preliminary results from its pivotal MARRS investigational device exemption (IDE) clinical trial at the Society for NeuroInterventional...
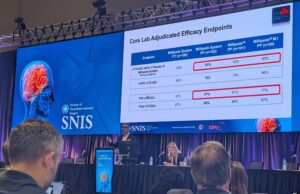
Physician feedback makes CEREGLIDE 71 a new gold-standard contender among stroke...
This advertorial, intended for readers outside the USA only, is sponsored by Johnson & Johnson MedTech Neurovascular.
“If you take all the good things about...
Gravity launches Supernova stent retriever and Neutron aspiration catheter for global...
Gravity Medical Technology has announced the launch of its next-generation stroke treatment devices: the Neutron aspiration catheter and the Supernova stent retriever. Early adoption...
Microvention announces publication of SOFAST clinical data
Microvention, a wholly owned subsidiary of Terumo Corporation, has today announced the publication of data from SOFAST—a prospective, multicentre US study assessing the efficacy...
Ceretrieve announces successful results in FIH study of stroke aspiration catheter
Ceretrieve has announced the successful results of the company's multicentre, single-arm study, showcasing the capabilities of its “state-of-the-art” aspiration catheter in stroke treatment.
The study—conducted across two...
Scientia announces US FDA approvals for new line of microfabricated neurovascular...
Scientia Vascular has announced the US Food and Drug Administration (FDA) clearance of two uniquely engineered catheters: the Plato 17, a dimethyl sulfoxide (DMSO)-compatible...
Q’Apel Medical launches new-generation Hippo aspiration system for stroke thrombectomy
Q’Apel Medical has launched a new-generation aspiration technology, the 072 Hippo aspiration system, which has been developed for patients suffering from a stroke due...
Perfuze enrols first stroke patient in MARRS pivotal trial of Millipede...
Perfuze has announced commencement of enrolment in its pivotal MARRS study—a multicentre trial set to evaluate the performance of aspiration thrombectomy with the company’s...
Infinity Neuro receives CE-mark approval for aspiration catheter to treat stroke
Infinity Neuro announced today that its Inspira aspiration catheters have received CE-mark approval and are now commercially available in Europe.
This is the first offering from...
MIVI Neuroscience names Waleed Brinjikji as new medical director
Next-generation neurointerventional medical device innovator MIVI Neuroscience has announced that Waleed Brinjikji (Mayo Clinic, Rochester, USA) has joined the company as its medical director.
“The...
Perfuze raises €22.5m in Series A funding for novel acute ischaemic...
Perfuze announced today that it has closed a €22.5 million Series A investment round—the proceeds from which will be used to drive the next...
Penumbra receives FDA clearance for RED 62 reperfusion catheter
Penumbra has announced US Food and Drug Administration (FDA) 510(k) clearance and commercial availability of the RED 62 reperfusion catheter—the latest addition to the...
HeMo Bioengineering receives China’s NMPA approval for Afentta aspiration catheter
HeMo Bioengineering, a Singapore-based medical device company with a focus on treating stroke patients, has announced that its Afentta intracranial thrombectomy aspiration catheter—a product...