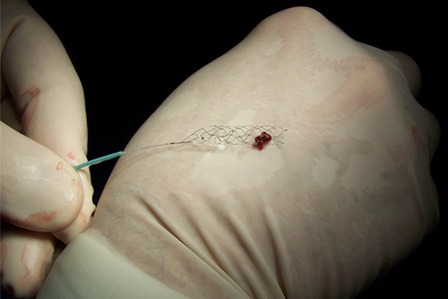
Stent retriever technology has been named by the Cleveland Clinic as a top medical innovation. It was part of a list of the top medical innovations for 2015, revealed at the Cleveland Clinic’s 2015 Medical Innovation Summit.
This news comes months after the American Heart Association/American Stroke Association (AHA/ASA) published new stroke treatment guidelines that recommend the use of stent retriever technology in conjunction with the current standard of care, IV-tPA, as a first-line treatment for eligible patients. The guidelines were based on a panel of experts’ analysis of the results from five global clinical trials published in The New England Journal of Medicine (NEJM) that found the addition of stent retriever technology, a surgical procedure that manually removes blood clots from the brain, to current medical therapy such as IV-tPA, has a therapeutic benefit over medical therapy alone. These trials, all of which studied the Solitaire device, showed that the addition of stent retriever technology reduced disability, improved neurological outcomes and increased the rate of return to functional independence in patients suffering stroke.
In a Medtronic press release, Brett Wall, president of the Neurovascular business, in the Restorative Therapies Group, at Medtronic, says, “Five global clinical trials have proven the Solitaire device as a major advancement in the treatment of stroke, reducing functional disability in patients. Having this technology recognised by the Cleveland Clinic’s prestigious medical innovations list as well as the new AHA/ASA guidelines has validated our belief in stent retrievers.”
The Solitaire stent retriever uses a micro-sized catheter to access arteries in the brain affected by stroke through an incision in the leg. Once delivered, the device helps to immediately restore blood flow and remove the blood clots causing the stroke.
The Solitaire FR stent retriever device received U.S. Food and Drug Administration 510(k) clearance in 2012 and Solitaire FR received CE Mark approval in 2009.









