Seven-year data from the Chinese CASSISS trial have, in the investigators’ view, provided “compelling evidence” that percutaneous transluminal angioplasty and stenting (PTAS) plus medical therapy does not confer additional benefits over medical therapy alone in patients with symptomatic, severe intracranial atherosclerotic stenosis. Peng Gao (Capital Medical University, Beijing, China) presented these findings at the 2024 European Stroke Organisation Conference (ESOC; 15–17 May, Basel, Switzerland), informing audiences that “these results underscore the importance of medical therapy as the cornerstone of long-term stroke prevention in this patient population”.
Gao began by noting that prior randomised trials, including SAMMPRIS and VISSIT—as well as initial follow-up data from CASSISS itself, published in 2022—have indicated no benefit when adding PTAS to medical therapy in symptomatic, severe intracranial atherosclerotic stenosis patients. However, he added that it “remains uncertain” whether PTAS can enable improved outcomes at five years or more. With three-year results having already been published, Gao and colleagues’ present analysis of CASSISS sought to shed further light on stenting versus medical therapy in these patients at an even longer follow-up timepoint.
CASSISS was a multicentre, open-label, randomised controlled trial conducted at eight centres across China, enrolling patients experiencing a transient ischaemic attack or ischaemic stroke with a modified Rankin scale (mRS) score of 0–2 attributed to severe intracranial atherosclerotic stenosis—meaning stenoses of 70–99%. These patients were then randomised 1:1 to either PTAS with the Wingspan stent (Stryker Neurovascular) plus medical therapy involving three months of dual antiplatelet therapy followed by single antiplatelet therapy thereafter, or medical therapy alone.
The trial’s primary endpoint was a composite outcome including stroke and death within 30 days post-enrolment, or stroke in the territory of the qualifying artery beyond 30 days. Between March 2014 and November 2016, a total of 380 patients were enrolled, with 188 being randomised to the PTAS group and 192 randomised to the medical therapy group. Some 358 patients were confirmed as eligible for final analyses and, of these, 237 (66.2%) completed the study’s seven-year follow-up.
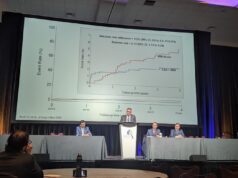
Regarding CASSISS’ primary endpoint, Gao reported no difference between the two study groups across the seven-year follow-up period (hazard ratio, 1.02; p=0.97), as 14.8% of 176 patients in the PTAS group and 14.3% of 182 patients in the medical therapy group experienced stroke/death within 30 days or qualifying-artery stroke beyond 30 days. The presenter also relayed no major differences between the PTAS and medical therapy groups concerning a number of secondary outcomes, including ipsilateral ischaemic stroke (14.8% vs 14.3%), disabling stroke or death (16.5% vs 14.3%), and death (9.1% vs 7.1%), after enrolment. Additional safety-related endpoints, such as rates of disabling ischaemic stroke, symptomatic intracranial haemorrhage, and both stroke-related and non-stroke-related death, were statistically similar between groups as well.
Intention-to-treat analyses of the two components of the primary endpoint revealed a trend towards a lower risk of stroke specifically in the territory of the qualifying artery post-30 days within the PTAS group (9.7%), compared to the medical therapy group (12.1%), but this observation did not reach statistical significance. This also contrasted with a slightly higher—but still statistically insignificant—rate of 30-day stroke/death in the PTAS group (5.1%) versus the medical therapy group (2.2%).
Gao noted some “inherent limitations” of the trial, including the fact it was conducted solely in Han Chinese patients and, as such, has “uncertain” generalisability to other populations. Also, CASSISS did not evaluate angioplasty alone—or other devices like drug-coated balloons and drug-eluting stents that are currently used off-label to treat intracranial atherosclerotic stenosis. Finally, Gao conceded that the trial may not fully reflect present-day clinical practice, as patients were treated in line with the 2014 American Stroke Association (ASA) guidelines, and many changes in stroke management are likely to have occurred in the years since then.
“Medical and interventional management have been improving in equal stride since the 2014 ASA guidelines,” Adam A Dmytriw (Massachusetts General Hospital, Boston, USA), one of the authors for the original publication of CASSISS in the Journal of the American Medical Association (JAMA) nearly two years ago, told NeuroNews. “It remains to be seen whether there is a select population of intracranial atherosclerotic stenosis patients who might benefit from stenting, and what tools we might need to make that selection.”