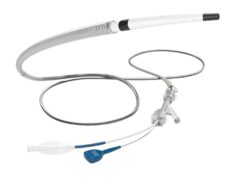
 Route 92 Medical has announced approval from the Australian Therapeutic Goods Administration (TGA) for its portfolio of innovative neurovascular interventional products intended to treat patients with acute ischaemic stroke.
Route 92 Medical has announced approval from the Australian Therapeutic Goods Administration (TGA) for its portfolio of innovative neurovascular interventional products intended to treat patients with acute ischaemic stroke.
Within this portfolio are the first 0.088-inch super bore reperfusion systems to be approved for use in Australia, including the FreeClimb 88 catheter powered by Tenzing technology and the Monopoint-based HiPoint 88 super-bore catheter, according to a Route 92 press release.
“The receipt of TGA approval is yet another step in our company’s journey to become a global leader in advancing neurovascular intervention,” said Tony Chou, Route 92’s founder and chief executive officer (CEO). “This registration gives us the ability to secure adoption of our products in a mature market with highly trained neurointerventionalists. As we innovate, simplify and improve neurovascular intervention, we plan to grow our footprint into additional geographies to address unmet needs.”
Route 92’s products are covered by a global portfolio of more than 140 patents. The TGA regulatory authorisation covers the majority of the company’s products, including:
- HiPoint 70/Tenzing 7 reperfusion system
- HiPoint 88/Tenzing 8 reperfusion system
- FreeClimb 70/Tenzing 7 reperfusion system
- FreeClimb 88/Tenzing 8 reperfusion system
- Base Camp sheath system
“We’ve had great success in our initial evaluation of Route 92 Medical’s stroke treatment portfolio,” said Hal Rice (Gold Coast University Hospital, Gold Coast, Australia). “It offers superb, enhanced delivery with the Tenzing system to navigate easily in the most tortuous of neurovasculature to quickly and efficiently reach the site of stroke vessel occlusion. The system offers robust capabilities that address a broad range of neurovascular intervention challenges, including efficiently navigating to and effectively aspirating stroke-causing clots to deliver rapid cerebral reperfusion and a high proportion of first-pass effect in cases we have recently treated.”
Route 92 recently showcased its technology at the Australian and New Zealand Society of Neuroradiology (ANZSNR) annual scientific meeting (27–29 March, Perth, Australia).