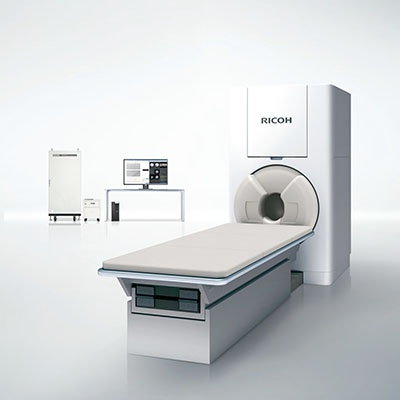
Ricoh has announced that the RICOH MEG Measurement System has received 510(k) clearance from the US Food and Drug Administration (FDA).
RICOH MEG, Ricoh’s magnetoencephalography system, is a non-invasive way to detect faint signals within the brain. It provides deeper measurement (hippocampus and hypothalamus), clearer signal using coaxial gradiometer sensors and greater patient comfort for better data acquisition.
“Ricoh is committed to helping advance research and treatment for neurological disorders, and MEG is another tool to help healthcare professionals and researchers measure brain activity,” says Scott Abelson, business development director, Medical Imaging, Ricoh USA.
Ricoh entered the medical device industry when it acquired a portion of a Yokogawa Electric business in 2016.













