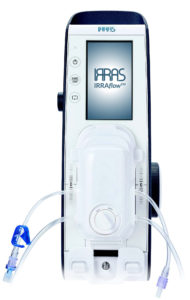
 Irras has announced a strategic collaboration with the neurosurgical team at Aarhus University Hospital in Aarhus, Denmark, to evaluate the use of the Irraflow during the treatment of intraventricular haemorrhage (IVH) and assessment of brain compliance.
Irras has announced a strategic collaboration with the neurosurgical team at Aarhus University Hospital in Aarhus, Denmark, to evaluate the use of the Irraflow during the treatment of intraventricular haemorrhage (IVH) and assessment of brain compliance.
According to a press release from the company, Anders Korshøj, associate professor of Neurosurgery at Aarhus University Hospital, will lead the collaboration that will unite specialists from the hospital’s neurocritical care team and data analytics experts from The Aarhus University Center for Digitalization, Big Data and Data Analytics. The collaboration will focus initially upon the use of the company’s Irraflow system to treat patients suffering from IVH. The facility’s use of Irraflow in the treatment of IVH will be prospectively compared to the use of traditional drainage treatments in the pending ACTIVE clinical study. Beyond this study, other clinical and product development projects are also being planned.
According to Irras, the Irraflow is the world’s first “irrigating intracranial drain” and its unique mechanism of action addresses the complications associated with the current methods of managing intracranial fluid by using a dual-lumen catheter that combines automated irrigation, controlled drainage, and continuous monitoring of intracranial pressure (ICP), all within one system.
“It is my belief that the system’s combination of automated irrigation and controlled drainage should contribute to better long-term outcomes for these critically ill patients,” said Korshøj. “Furthermore, we believe that the system’s ability to measure pressure immediately after fluid movement is uniquely capable of measuring real-time intracranial compliance, and I am excited to partner with Irras to explore these hypotheses in greater detail.”
Traditional treatment options for IVH patients generally utilise an external ventricular drainage (EVD) system, which is a passive approach that relies solely on gravity to facilitate drainage, states Irras. Although an EVD is currently the most common treatment option for intracranial bleeding or elevated ICP, the technology is associated with several well-known complications such as catheter blockage, infections, and incomplete drainage, the company claims. All of these complications can negatively impact patient outcome, which can subsequently increase the length of time needed in the hospital and the overall cost of care.
“This collaboration with Aarhus University Hospital represents an important component of our launch strategy for Irraflow,” said Will Martin, president and chief commercial officer of Irras. “There is a compelling need in markets around the globe for transformative technologies, such as Irraflow, to treat patients with intracranial bleeding, and we believe that our collaboration with Aarhus University Hospital to collect clinical data will advance the treatment of IVH and also accelerate the product’s awareness to a wider group of neurocritical care specialists.”







