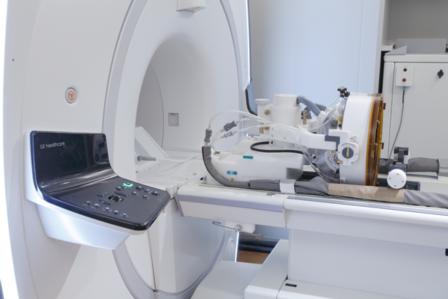
Insightec has announced that it has submitted a pre-market approval application (PMA) to the US Food and Drug Administration (FDA) for its Exablate Neuro treatment of essential tremor.
Exablate Neuro uses high intensity focused ultrasound to thermally ablate the target tissue and continuous MRI to visualise the anatomy, plan treatment and monitor treatment outcomes. In the brain, MR guided focused ultrasound (MRgFUS) is used to perform a non-invasive thalamotomy through intact skull to relieve tremor in patients with essential tremor.
Exablate Neuro noninvasive ablation was investigated as a treatment alternative for these patients. The PMA submission was based on a two arm randomised controlled trial comparing patients undergoing Exablate MRgFUS treatment with patients undergoing a placebo treatment. Patients in the placebo treatment arm were later allowed to undergo an Exablate Neuro treatment. The FDA granted the Expedited Access Pathway designation.
“This is another major achievement, our third clinical indication submitted for FDA approval and the first PMA for Neurosurgery,” said Kobi Vortman, chief executive officer and founder of Insightec. “This represents our continued commitment to transform MRgFUS from an investigational technology to a standard of care treatment option for neurological patients,” he concluded.
“This is the first transcranial MRgFUS treatment being reviewed for FDA approval. It follows years of development with significant technological breakthroughs,” said Eyal Zadicario Insightec’s vice president of R&D and Neuro Program. “We believe that this is only the starting point in many neurosurgery developments to come our way in the coming future,” he concluded.
Funding for the study has been provided by the Focused Ultrasound Foundation and BIRD (US-Israel Binational Industry R&D) and Insightec.
Participating medical sites: University of Virginia, Stanford University Hospital, University of Maryland, Brigham and Women’s Hospital, Swedish Medical Center, Yonsei University College Of Medicine (Korea), Sunnybrook Hospital, Tokyo Women’s Medical University, Tremor Research Group – Core Lab.








