The first patients have been enrolled in the TIGER (Treatment with Intent to Generate Reperfusion) study. This is a multicentre study of the performance of TIGERTRIEVER (Rapid Medical), a thrombectomy device for the acute treatment of ischaemic stroke.
The TIGER study is an investigative device exemption (IDE) clinical study for the purpose of supporting Rapid Medical’s 510(k) submission to the US Food and Drug Administration to obtain clearance to market the device in the USA.
The study will take place in up to 25 stroke centres throughout the USA. Neurologists, Jeffrey Saver, UCLA Medical Center in Los Angeles, and Rishi Gupta, director, Neurocritical Care at WellStar Health System, Georgia, are the principal investigators of the study.
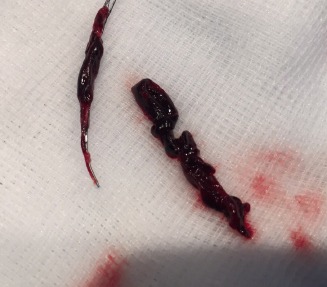
More than a thousand patients have been commercially treated with the TIGERTRIEVER in Europe, and it is the subject of a post-marketing registry in several European countries. The TIGERTRIEVER is a fully-visible, controllable stent retriever that is adjusted to fit the dimensions of a blocked blood vessel causing acute ischaemic stroke.
The initial cases in the IDE study were performed by Gupta and Ahmad Khaldi, the director of Cerebrovascular Neurosurgery at Wellstar Medical. “We are pleased to have enrolled the first patients and to have a leadership role in the TIGER study. Stent retrievers are now the gold standard for treating ischaemic stroke, and TIGERTRIEVER is a new generation stent retriever. Its unique design will hopefully show that it addresses the limitations of current devices and will improve patient outcomes,” states Gupta.













