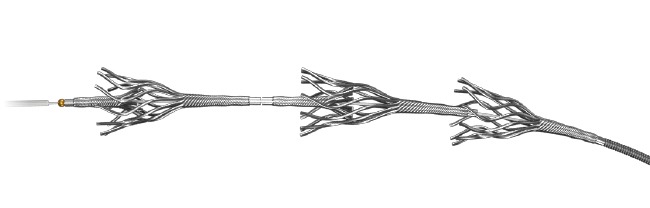
Amnis Therapeutics has received approval to perform a first-in-human clinical trial with its neuro thrombectomy device, the Golden Retriever. The approval was received from the Karolinska Institute (Stockholm, Sweden).
Tommy Andersson, Karolinska University Hospital, Stockholm, Sweden, will be the trial’s principal investigator. The trial will include 60 patients suffering from acute ischaemic stroke, with a large intracerebral vessel occlusion.
The company will start recruiting patients shortly and expects the trial to conclude during Q1, 2018. The physicians will use the Golden Retriever to extract the blood clot and restore blood flow in the brain. The device’s small size and extreme flexibility enable easy access and fast extraction of any clot, regardless of the clot’s size or consistency.
The trial will be the basis for submission of CE mark approval in order to market the device in CE countries. The company is in the process of adding more clinical centres to be included in the trial, including centres in Barcelona, Spain and in Israel.
Aviv Lotan, CEO of Amnis Therapeutics, says, “We are happy to start the clinical phase of testing the Golden Retriever, which is the last phase, prior to receiving a CE mark. We believe that the Golden Retriever’s attributes (small diameter, deployment mechanism, ease of access and use), suggest significant advantages over existing products for neuro thrombectomy.”












