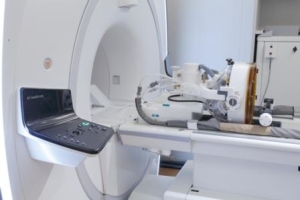
 The US Food and Drug Administration has approved the first focused ultrasound device to treat essential tremor in patients who have not responded to medication. ExAblate Neuro from Insightec uses magnetic resonance (MR) images taken during the procedure to deliver focused ultrasound to destroy brain tissue in a tiny area thought to be responsible for causing tremors.
The US Food and Drug Administration has approved the first focused ultrasound device to treat essential tremor in patients who have not responded to medication. ExAblate Neuro from Insightec uses magnetic resonance (MR) images taken during the procedure to deliver focused ultrasound to destroy brain tissue in a tiny area thought to be responsible for causing tremors.
“Patients with essential tremor who have not seen improvement with medication now have a new treatment option that could help them to avoid more invasive surgical treatments,” says Carlos Peña, director of the division of neurological and physical medicine devices in the FDA’s Center for Devices and Radiological Health. “As with other treatments for essential tremor, this new device is not a cure but could help patients enjoy a better quality of life.”
To determine if the ExAblate Neuro treatment is appropriate, patients should first have MR and computerised tomography (CT) scans. Those undergoing treatment with the MRI-guided device lie in an MRI scanner that takes images to help a doctor identify the targeted area in the brain’s thalamus for treatment. Treatment with transcranial focused ultrasound energy is administered with incremental increases in energy until patients achieve a reduction of tremor. Patients are awake and responsive during the entire treatment.
Data supporting the safety and effectiveness of the device system included a double-blind control trial involving 76 patients with essential tremor who had not responded to medication therapy. Fifty-six of the patients were randomly selected to receive the ExAblate Neuro treatment and 20 received a fake treatment. Patients in the control group were able to cross over into the treatment group three months later.
Patients treated with the ExAblate Neuro showed nearly a 50% improvement in their tremors and motor function (composite tremor/motor function score) three months after treatment compared to their baseline score. Patients in the control group had no improvement, and some experienced a slight worsening after the sham procedure before they crossed over into the treatment group. At 12 months post-procedure, the treatment group retained a 40% improvement in these scores compared to baseline.
Adverse events for the ExAblate Neuro are consistent with those reported for thalamotomy surgery, including numbness/tingling of the fingers, headache, imbalance/unsteadiness, loss of control of body movements (ataxia) or gait disturbance. Other side effects identified as possibly related to treatment with MR-guided focused ultrasound treatments include tissue damage in an area other than the treatment area, haemorrhage in the treated area requiring emergency treatment, skin burns with ulceration of the skin, skin retraction and scar formation and blood clots.
The ExAblate Neuro treatment is contraindicated for patients who cannot have MR imaging, including those who have a non-MRI compatible implanted metallic device, such as a cardiac pacemaker, those with allergies to MR contrast agents or those with body size limitations for MR.








