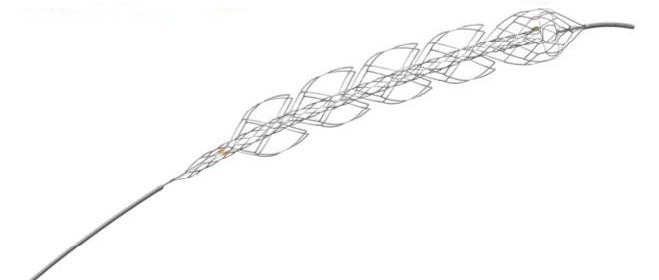
Enrolment is now complete in the ARISE II clinical trial which is assessing the safety and effectiveness of the EmboTrap II revascularisation device (Neuravi), an advanced stent retriever platform for the treatment of acute ischaemic stroke.
Data from ARISE II (Analysis of revascularization in ischemic stroke with EmboTrap) will be submitted as part of an application to US Food and Drug Administration (FDA) for market clearance of the device in the United States.
The ARISE II study enrolled 228 patients in 19 enrolling sites across the United States and Europe. Sam Zaidat, Stroke and Neuroscience medical director of St Vincent Mercy Hospital in Toledo, USA, is the study’s principal investigator. Tommy Andersson of the Karolinska Institute in Stockholm, Sweden, is the European principal investigator of the study.
“The value of stent retrievers has been demonstrated by multiple positive trials. Now our focus has moved on to further refinements of mechanical thrombectomy that will yield better patient outcomes,” says Zaidat. “International cooperation has been excellent. I am excited to be a part of the process to provide physicians and patients in the United States with access to the latest technology.”
The study enrolled ahead of schedule, with enrolment led by Hormozd Bozorgchami’s team at Oregon Health Sciences University in Portland, USA and Marc Ribo’s team at Vall d’Hebron in Barcelona, Spain.
“Evaluating new technology is an important part of advancing stroke treatment and we are enthusiastic about our experience in the ARISE II trial,” says Raul Nogueira, director of Neuroendovascular Service at Grady Memorial Hospital at Emory University in Atlanta, USA, where the final US patient was enrolled. “As we continue to learn more about the clots that cause stroke, it is important that we have the best tools available to treat those occlusions.”
According to Neuravi, the EmboTrap platform is designed to restore blood flow to the brain by retrieving and retaining clot with a proprietary dual-layer stent-like structure.









