Suzie Marshall
New devices for endovascular thrombectomy discussed at CIRSE
In a session of the 2020 Cardiovascular and Radiological Society of Europe (CIRSE) meeting (12–15 September, virtual) focused on the hottest news in the...
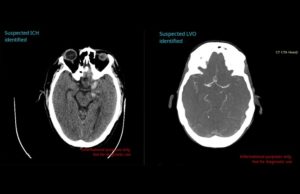
Avicenna.AI secures FDA clearance for its CINA Head neurovascular imaging artificial...
Medical imaging artificial intelligence (AI) specialist Avicenna.AI has announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for its...
CMSS “strongly urges action” to ensure all frontline healthcare professionals are...
In a new statement published on 2 April 2020, all 45 societies represented by the US Council of Medical Specialty Societies (CMSS)—over 800,000 physicians—emphatically...
NeuroPace RNS System receives FDA approval for MRI Labeling, allowing thousands...
NeuroPace has announced that its RNS System has received US Food and Drug Administration (FDA) approval of MRI labeling for the RNS System, expanding...
Medtronic issues urgent field safety notice for some Pipeline Flex embolisation...
Medtronic has issued an urgent field safety notice to warn on specific production lots of its Pipeline Flex embolisation devices due to the potential...
NeuroVasc secures US$34 million in funding to support clinical trials and...
NeuroVasc Technologies has entered into a strategic partnership with the Wego Group that includes US$34 million in funding to support the company’s product portfolio...
Ghana making giant strides: Neurointerventional practice in sub-Saharan Africa
Since founding the Ghana Society of Interventional Radiologists in November 2015, Benjamin Dabo Sarkodie has been working to establish the offering of minimally invasive,...
LivaNova enrols first patient in RECOVER clinical study
LivaNova has announced the first patient enrolled in their RECOVER trial, a prospective, multi-centre, randomised controlled blinded trial demonstrating the safety and effectiveness of...
Surface modification technology could revolutionise ruptured aneurysm treatment globally
Laurent Pierot (Reims, France) talks to BLearning Neuro at ESMINT 2019 (European Society of Minimally Invasive Neurological Therapy; 4–6 September; Nice, France) about the HPC (Hydrophilic Polymer Coating)...
MRI Interventions reaches ClearPoint milestone
MRI Interventions said its ClearPoint Neuro navigation system has been used in 3,000 neurosurgical procedures.
The milestone procedure was completed at the University of California,...
Siemens Healthineers to acquire Corindus Vascular Robotics
Siemens Healthineers will acquire all issued and outstanding shares of common stock of Corindus Vascular Robotics, Corindus announced this month.
The cash transaction is US$4.28...
Scott Gottlieb resigns as head of US FDA
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the...
Acute ischaemic stroke care continuum: With stroke patients every step of...
The fact that endovascular therapy is now well established for patients with acute ischaemic stroke has changed the face of stroke treatment forever. The last five...
US government shutdown disrupting FDA work
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new...
Balt announces receiving CE mark for Magic Glue, a next generation...
Balt International has announced receiving the CE mark for Magic Glue, its next generation of cyanoacrylate liquid embolic agent. Magic Glue is indicated for...
Addressing patient variability and the dynamic nature of pain: The value...
With a series of new products this year and the acquisition of Cosman Medical’s radiofrequency portfolio, Boston Scientific continues to drive forward as a...
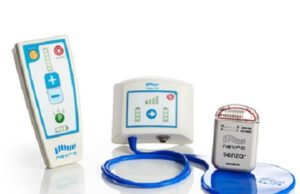
Nevro receives TGA approval for Senza II spinal cord stimulation system...
The Therapeutic Goods Administration (TGA) has granted approval to Nevro for its next-generation Senza II spinal cord stimulation system delivering the company’s proprietary HF10...
International neurointerventional societies outline new criteria for facilities that treat stroke
Thirteen neurointerventional societies have released new guidelines outlining the criteria for Level 1, 2 and 3 stroke centres that provide acute ischemic stroke interventions...
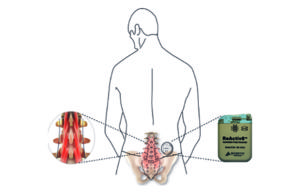
Mainstay Medical announces the completion of all implants in the ReActiv8-B...
Mainstay Medical International, a medical device company focused on bringing to market ReActiv8, an implantable neurostimulation system to treat chronic low back pain, announces...
NSI-566 stem cell therapy confers neurological improvement in spinal cord injury...
Intraspinal injections of human spinal cord-derived neural stem cells are well tolerated in a Phase I study of four patients with thoracic (T2–T12) ASIA-A...
FDA releases final rule on acceptance of data from clinical investigations...
The Food and Drug Administration (FDA) has today issued the final rule on “human subject protection; acceptance of data from clinical investigations for medical...