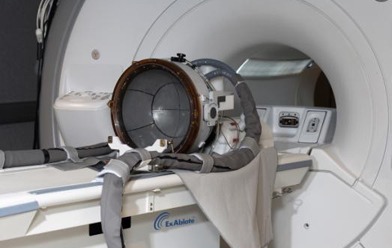
Elbit Imaging, a shareholder in InSightec, has announced that the company’s Exablate Neuro platform has been approved for the treatment of movement, pain and behavioural disorders by the Korean Ministry of Food and Drug Safety.
Insightec’s Exablate Neuro platform is designed to be a non-invasive treatment alternative. It combines focused ultrasound to lesion targeted tissue deep in the brain, and magnetic resonance imaging (MRI) to guide the ultrasound waves and to provide real-time feedback on treatment progress and outcome.
This regulatory approval allows Korean patients suffering from neurological disorders which cause significant disability access to a treatment option that does not require open surgery.
The Company holds approximately 89.9% of the share capital of Elbit Medical Technologies, which, in turn, holds approximately 29.6% of the share capital in InSightec.








