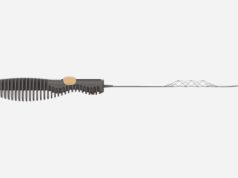
The first patient has been enrolled in the TIGERTRIEVR registry at the Cantonal Hospital of Lucerne, Switzerland. The Tigertriever (Rapid Medical) is a one-of-a-kind, fully-visible, controllable stent retriever that is adjusted to perfectly fit the dimensions of a blocked blood vessel causing acute ischaemic stroke.
The TIGERTRIEVER registry is a European multicentre registry that will enrol patients from leading centres in France and Switzerland. It is the first registry that is designed to demonstrate the benefits of the Tigertriever in real-life use.
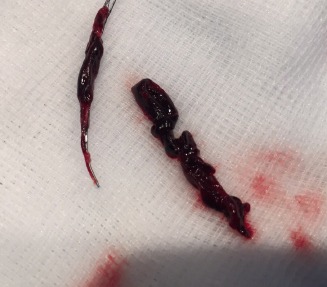
Alexander von Hessling, Division of Neuroradiology, Cantonal Hospital of Lucerne, Switzerland described the first procedure in the registry: “An 87-year-old male was admitted to the hospital with an acute stroke, suffering from severe aphasia and right sided hemiplegia. The patient was treated with the Tigertriever which removed a large clot from his left internal carotid artery in a single attempt. The procedure went very well and took 19 minutes, including placement of a carotid stent due to a severe carotid stenosis. The patient recovered quickly and completely and was even able to sign his consent to be enrolled in this registry study one hour after finishing the procedure. This is a very exciting and promising device: It is controllable and fully visible. We are happy to have enrolled the first patient and to have a leadership role in this registry.”
According to a company release, in addition to the post marketing registry, Rapid Medical plans to initiate a prospective multicentre clinical study for US FDA clearance for the Tigertriever. The TIGER study is planned to start enrolling patients in leading centres in the USA, Europe and Israel during the first half of 2018.