The Woven EndoBridge (WEB) 17 device (Microvention/Terumo Corporation) has demonstrated positive efficacy and safety outcomes in the prospective, multicentre CLEVER study, indicating its utility for intrasaccular embolisation across ruptured and unruptured intracranial aneurysms. Twelve-month results from the study were recently delivered by Laurent Spelle (Bicêtre Hospital, Paris, France) at the LINNC Americas Seminar (16–17 March, Miami, USA).
The CLEVER study was set up to assess whether—despite its lower profile in comparison to prior device iterations—the newer-generation WEB 17 system maintains efficacy and safety in the treatment of bifurcation aneurysms, Spelle reported. It was conducted at 17 clinical sites spanning Finland, France, Germany, Hungary and the UK, with Spelle being one of three trial coordinators alongside Christophe Cognard (Toulouse University Hospital, Toulouse, France) and István Szikora (National Institute of Mental Health, Neurology and Neurosurgery, Budapest, Hungary).
A total of 163 patients (mean age 58.1 years, 68% female) including 103 with unruptured aneurysms and 60 with ruptured aneurysms, none of which had previously been treated, were enrolled from June 2019 to February 2021. CLEVER’s primary safety endpoint was death of non-accidental cause/any major stroke ≤30 days after treatment, or death/major ipsilateral stroke of neurologic cause from day 31 to one year after treatment. Its primary efficacy endpoint—evaluated at James Byrne’s (University of Oxford, Oxford, UK) core lab—was adequate occlusion without retreatment at one year.
Outlining the study results, Spelle highlighted that there were no treatment failures with WEB 17 across all 163 aneurysms, while adjunctive devices—primarily balloons—were required in just nine unruptured (8.7%) and seven ruptured (11.7%) cases.
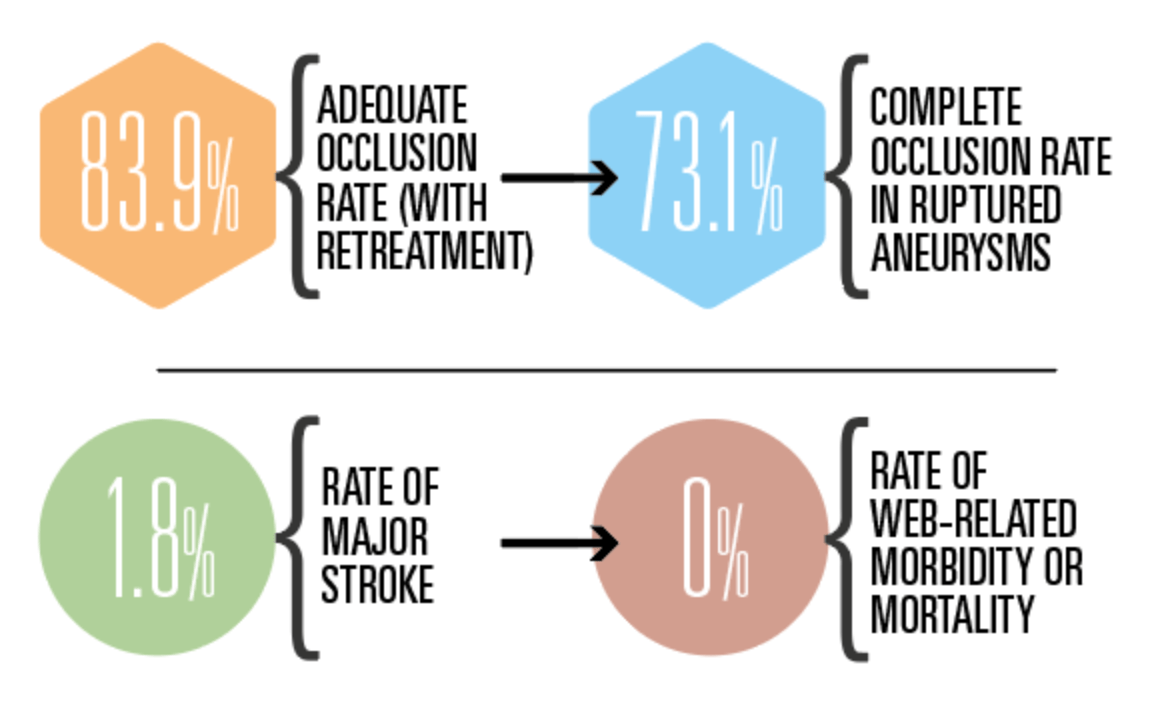
Based on clinical event adjudication, CLEVER met its key safety endpoint, with three patients (1.8%) suffering a major stroke and zero deaths due to a neurologic cause occurring within one year post-treatment. Touching on the study’s secondary safety endpoints, Spelle also noted a 2% rate of morbidity (defined as modified Rankin scale [mRS] >2) and a 0.6% overall mortality rate at 12 months, as well as one perioperative bleeding event and no rebleeding in patients with ruptured aneurysms, and one haemorrhagic complication in patients with unruptured aneurysms. He further detailed that none of the haemorrhagic complications seen within one year had any clinical consequences, and that no WEB-related morbidity or mortality was observed.
Moving on to discuss the study’s primary efficacy endpoint—ultimately assessed in 146 patients—Spelle reported an 82.2% rate of target aneurysm adequate occlusion (complete occlusion or neck remnant) without retreatment at one year. Regarding secondary efficacy outcomes, he stated that four of 152 patients (2.6%) required retreatments at 12 months, and pointed to complete occlusion (62.9%) and neck remnant (21%) rates on the three-grade Raymond-Roy occlusion classification (RROC) scale, resulting in an 83.9% adequate occlusion rate when allowing for retreated aneurysms.
Finally, the speaker relayed 73.1% complete occlusion and 86.6% adequate occlusion rates as per RROC, and one retreatment, in the study’s ruptured aneurysm subgroup. Spelle then drew comparisons between CLEVER and the CLARYS study, which assessed the original, larger WEB device in ruptured aneurysms. He concluded that, “along its evolution, the WEB device remains safe and efficient” in such cases, citing similar maximum aneurysm sac widths in CLARYS and CLEVER’s ruptured subgroup, as well as lower rates of morbidity and mortality observed in the latter patient cohort.