
By Tommy Andersson
Recently no less than five randomised controlled trials showed superiority for intra-arterial treatment added to intravenous thrombolysis (IVT) of large artery stroke in the anterior circulation as compared to stand-alone IVT1–5. This obvious breakthrough is more than welcomed, but it is also clear from these studies that challenges remain, not least for the neurointerventionalists. In all the studies, the revascularisation percentage was far from 100% (Figure 1); sometimes it is quite impossible to remove the thrombus in a safe and efficient way. Further, even if perfect revascularisation is achieved, the percentage of patients having a good outcome is much less. Such so-called futile revascularisation has probably many reasons, but one of them may be a prolonged procedure time.
So, if it is impossible to achieve revascularisation, or, if it takes too long resulting in infarct growth while the patient is on the angio table, can this be related to clot properties? And further, may other clot properties as well as reperfusion-related pathophysiological incidents make our efforts fruitless and jeopardise a good outcome for our patients?
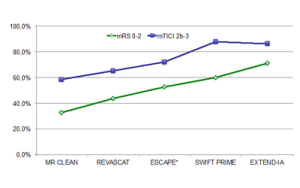
Clot properties may influence the revascularisation rate
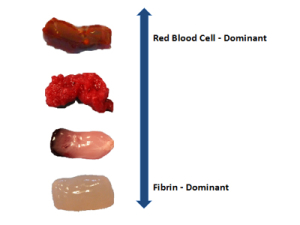
A thromboembolus contains many different substances, but one very important factor seems to be the relative content of fibrin6–7. In research that we have conducted in cooperation with Neuravi, we found that the proportion of fibrin versus red blood cells in a clot substantially alters its physical properties and subsequently the possibility to extract it (Figure 2). A mature, fibrin-rich clot is firm, tough and sticky, and therefore much less likely to deform. With this follows the obvious risk of it being difficult to remove with conventional stent retrievers or with aspiration alone. In contrast, clots rich in red blood cells are soft, friable and slippery which means that they may be easier to remove but instead more prone to embolisation into the same or a previously unaffected territory. Flow-arrest utilising a balloon-guide catheter becomes crucial in this context to reduce the risk of embolisation during thrombectomy, which we have found in our work both clinically and in the laboratory. The challenge of removing a range of clot types may require a technology that interacts with the clot differently than current devices. The EmboTrap device (from Neuravi) has been designed to encapsulate and remove different types of clots and has proven capable of doing that efficiently in our series of thrombectomy patients.
The clot changes its properties on site
Not only are features of the clot variable, they also change during the occlusion process. From our studies, it was clear that the longer the clot remained in place, the more sticky and difficult to remove it became. When the clot was compressed by the water-hammer effect of the systemic blood pressure, it changed its properties to become more compacted and difficult to remove. Further, clot properties were altered by each of our attempts to remove them. For every failed thrombectomy attempt, the clot became more difficult to remove.
Other clot properties and reperfusion injury
Clots obviously contain many other substances beside fibrin and red blood cells. We have an active research collaboration with KU Leuven Campus Kulak in Kortrijk, Belgium, where the group at the Laboratory for Thrombosis Research has experimentally shown that clots collected from our thrombectomised patients contain various levels of von Willebrand factor. This is a large multimeric plasma glycoprotein that, together with fibrin, links platelets together and thereby may make the clot more organised and tougher. Destabilising the clot by counteracting the creation of large and more active multimers may prove to be beneficial both for intravenous thrombolysis and for thrombectomy8–9.
In this context, another, perhaps not yet fully appreciated, reason for futile revascularisation (ie. bad clinical outcome in spite of technical success) is injury caused by the reperfusion itself. Reperfusion into an ischaemic territory may indeed also activate von Willebrand factor, which in turn leads to thrombus formation in small arteries downstream. Blocking the function of von Willebrand factor was shown to significantly reduce stroke reperfusion injury in mice9–10. In clinical cases where everything seemingly goes very quickly and smoothly yet there is futile revascularisation, we tend to blame our selection process for the bad outcome. But perhaps this kind of secondary injury deprives us, and especially the patient, of a good outcome.
 Implications for practice and future research
Implications for practice and future research
In addition to shortening the times from onset to imaging and from imaging to groin puncture, we need to develop techniques and devices that make it possible to more safely and efficiently remove the clot. Ideally, this should be possible with one to two attempts and the total time of the procedure, from groin puncture to revascularisation, should not exceed 15–20 minutes. The final TICI-score is less important if the procedure takes 90 minutes!
In our experience clinically and in our laboratory investigations, the use of a balloon-guide catheter with concomitant flow-arrest along with deliberate, optimal positioning of the devices have proved to be crucial in minimising the number of attempts required to revascularise the patient. Further, future thrombectomy devices should be designed to be able to manage all types of clots, or, there should be different devices specifically designed for a certain type of clot. We need to understand more the full spectrum of clot properties to try to find ways to more efficiently extract them. Finally, we need more research into the fascinating field of reperfusion injury to learn how to avoid and counteract this possible mechanism that results in bad outcomes.
Tommy Andersson is a neurosurgeon and neuroradiologist at AZ Groeninge Kortrijk, Belgium and Karolinska University Hospital, Stockholm, Sweden
References:
- Berkhemer OA et al, A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20.
- Goyal M et al, Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-1030.
- Campbell BC et al, Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-1018.
- Saver JL et al, Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372:2285-2295.
- Jovin TG et al, Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-2306.
- Liebeskind DS et al, CT and MRI Early Vessel Signs Reflect Clot Composition in Acute Stroke. Stroke 2011;42:1237-1243.
- Cline B et al, O-027 Pathological analysis of extracted clots in embolectomy patients with acute ischaemic stroke. J Neurointervent Surg. 2013;5:A15-A16.
- Denorme F et al, ADAMTS13 destabilizes thrombi in a mouse model of thrombotic focal cerebral ischemia. J Thromb Haemost. 2015;13(Suppl 2):694–695.
- De Meyer SF et al, Von Willebrand factor: an emerging target in stroke therapy. Stroke 2012;43:599–606.
- De Meyer SF et al, Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice–brief report. Arteriosclerosis, Thrombosis, and Vascular Biology 2010;30:1949–1951.











