Tag: SNIS 2025
Aspiration thrombectomy produces notably lower sICH and vasospasm rates versus stent...
Analyses of a large US registry comparing first-line stroke thrombectomy approaches for medium-vessel occlusion (MeVO) stroke have shown that direct aspiration is associated with...
More research needed to explore potential racial, gender and socioeconomic differences...
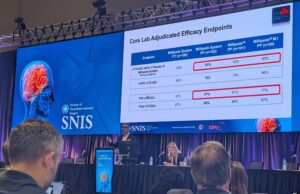
Research presented recently at the Society of NeuroInterventional Surgery (SNIS) annual meeting (14–18 July, Nashville, USA)—across three studies—has explored differences in treatment and recovery...
Pregnancy linked to increased rupture risk in brain AVM patients, meta-analysis...
At this year’s Society of NeuroInterventional Surgery (SNIS) annual meeting (14–18 July, Nashville, USA), researchers presented findings indicating the extent to which pregnancy can...
Six-month results find positive technical and clinical outcomes in robotic-assisted aneurysm...
Six-month follow-up results from a “pioneering” trial of robotic-assisted neuroendovascular aneurysm embolisation using the CorPath GRX system (Siemens Healthineers) were presented by Vitor Pereira...
Diabetes drugs like Ozempic may help mitigate effects of stroke and...
GLP-1 inhibitors like semaglutide (Ozempic)—commonly prescribed diabetes medications that lower blood sugar levels and often cause weight loss—may be able to mitigate against strokes...
EMBO-02 study indicates rapid cSDH resorption and pain-free treatments with NeoCast...
Arsenal Medical has announced late-breaking data from the EMBO-02 clinical study evaluating NeoCast—a next-generation, shear-responsive liquid embolic agent—in the treatment of patients with chronic...
Late-breaking MARRS trial data reveal 61% first-pass effect rate with Perfuze’s...
Perfuze has announced the presentation of positive preliminary results from its pivotal MARRS investigational device exemption (IDE) clinical trial at the Society for NeuroInterventional...
Imperative announces new data supporting use of Zoom system in M2...
Imperative Care has today announced late-breaking data from the Imperative trial evaluating aspiration thrombectomy via the Zoom system in stroke patients with M2 occlusions,...
Revalesio to present Phase 3 study design and RESCUE trial post-hoc...
Revalesio has announced that two abstracts featuring its investigational treatment RNS60 in acute ischaemic stroke have been accepted for oral presentation at the Society...
Terumo unveils new Sofia 88 data demonstrating “strong performance” versus other...
Terumo Neuro has unveiled new, real-world US physician preference data for the Sofia 88 neurovascular support catheter at the 2025 Society of NeuroInterventional Surgery...
Route 92 announces new data on HiPoint reperfusion system alongside SUMMIT...
Route 92 Medical has today announced results from an independent, real-world study evaluating reperfusion outcomes following use of the company’s HiPoint reperfusion system—described by...
Q’Apel announces US FDA clearance of Zebra neurovascular access system, prepares...
Q'Apel Medical has announced the receipt of US Food and Drug Administration (FDA) clearance for its Zebra neurovascular access system, with a full US...