Tag: DRG stimulation
Abbott introduces new delivery system to streamline electrode placement during DRG...
Abbott has announced the launch of its neuromodulation business’ next-generation delivery system, which will be used to streamline the implantation process for electrodes used...
Abbott gains expanded MRI labelling from US FDA for Proclaim DRG...
Abbott has announced that the US Food and Drug Administration (FDA) has approved expanded magnetic resonance imaging (MRI) labelling for its dorsal root ganglion...
First SynerFuse procedure performed by solo surgeon in two-level spinal fusion...
SynerFuse has announced that—for the first time in the world, it claims—a solo spine surgeon has performed the SynerFuse procedure in a two-level spinal...
SynerFuse announces presentation of IDE proof-of-concept study data
SynerFuse has announced that Michael Park (University of Minnesota, Minneapolis, USA)—principal investigator for the company’s investigational device exemption (IDE) proof-of-concept study—presented data from the study’s...
SynerFuse announces “milestone” solo spine surgeon procedure in DRG stimulation study
Justin Zenanko, the CEO of SynerFuse, announced today that—in what the company says is a world-first—a solo spine surgeon has performed the “groundbreaking” SynerFuse...
Intermittent stimulation of the DRG produces comparable chronic pain results to...
Intermittent dorsal root ganglion stimulation (DRG-S) has been shown to produce comparable results to continuous stimulation across a two-week period, as reported in Neuromodulation:...
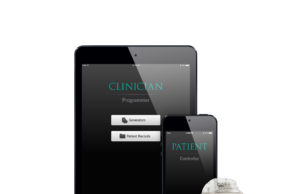
Abbott upgrades NeuroSphere myPath app for patient monitoring in neuromodulation trials
Abbott today announced that it has launched an upgraded version of its NeuroSphere myPath digital health app with enhanced functionality intended to help doctors...
Lower frequencies may improve outcomes of pain treatment with dorsal root...
Lower frequencies of dorsal root ganglion stimulation (DRG-S) may be more effective in the treatment of neuropathic pain—as per a late-breaking presentation from Guilherme...
Updated medical policy expands patient access to Abbott’s DRG stimulation technology
Abbott announced today that UnitedHealthcare (UHC)—the largest private health insurance company in the USA—has updated its ‘Implanted electrical stimulator for spinal cord’ medical policy...
First patient implanted in integrated spinal fusion and DRG stimulation proof-of-concept...
SynerFuse has announced the first implantation in the company's proof-of-concept study evaluating the safety and tolerability of simultaneously implanting spinal fusion hardware and a dorsal...
Dorsal root ganglion stimulation evokes motor responses in patients with complete...
Bilateral L4 dorsal root ganglion (DRG) stimulation has been shown to evoke strong and reproducible motor responses in the upper leg in patients with...
Pooled analysis finds dorsal root ganglion stimulation “safe and effective” for...
Dorsal root ganglion (DRG) stimulation is a safe and effective therapy for multiple chronic pain disorders, concludes a pooled analysis published in Neuromodulation. The...
The top 10 takeaways from “The Neuromodulation Appropriateness Consensus Committee on...
In 2018, the International Neuromodulation Society (INS) project on Consensus Conferences published “The Neuromodulation Appropriateness Consensus Committee on Best Practices for Dorsal Root Ganglion...
New approvals allow chronic pain sufferers to try Abbott’s non-opioid pain...
Abbott has announced the launch of the new dorsal root ganglion (DRG) Invisible Trial System, which is approved by the US Food and Drug...
Evidence in favour of DRG stimulation builds with real-world data
The real-world data collected in the PREDICT study has added to the evidence in favour of dorsal root ganglion (DRG) stimulation for the management...
Proclaim DRG Neurostimulation System introduced in Europe
Abbott has announced the European launch of the new Proclaim DRG Neurostimulation System, designed to deliver dorsal root ganglion (DRG) stimulation to patients suffering...
ACCURATE 12-month data published in Pain
New data published in the January edition of Pain has confirmed the superiority of dorsal root ganglion (DRG) stimulation therapy (from Abbott) over traditional...
Southeast Pain Care uses new Axium neurostimulator for chronic pain
Southeast Pain Care is one of the first facilities in North Carolina, USA, to use a new neurostimulation device designed to relieve the sensation...
First US commercial implants of Axium DRG system announced
St Jude Medical has announced the US launch and first post-approval implants of the St Jude Medical Axium Neurostimulator System for dorsal root ganglion (DRG) stimulation...
St. Jude Medical: Returning innovation to chronic pain treatment
With a series of acquisitions and recent product approvals, St. Jude Medical has further solidified its place as a leading provider of treatment options...
St Jude Medical completes acquisition of Spinal Modulation
Now complete, the acquisition of Spinal Modulation adds DRG stimulation technology to the St Jude Medical chronic pain portfolio.
St Jude Medical announces intent to acquire Spinal Modulation
Following the completion of this acquisition, St Jude Medical will become the only medical device manufacturer to offer radiofrequency ablation (RFA), spinal cord stimulation (SCS) and dorsal root ganglion (DRG) stimulation therapy solutions for the treatment of chronic pain.