The US Food and Drug Administration (FDA) has granted breakthrough device designation to the Stentrode brain-computer interface for its fully-implantable medical device that can translate brain activity or stimulate the nervous system from the inside of a blood vessel, without the need for open brain surgery.
The device, manufactured by Synchronhas already been implanted in patients with upper-limb paralysis and is currently being evaluated for its ability to enable patients with paralysis to regain functional independence by control of digital devices through thought alone.
The breakthrough devices programme is a voluntary programme for certain medical devices and certain device-led products, that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions, with the aim to provide timely access to these devices and lower the burden required for Medicare reimbursement.
“As this is a first-of-its-kind device, we look forward to working closely with the FDA to prioritise development of the Stentrode and ensure access for patients with paralysis, as well as lay the groundwork for future indications for brain-computer interfaces,” said interventional neurologist Thomas Oxley (Mount Sinai Health System, New York, USA), who is also CEO of Synchron.
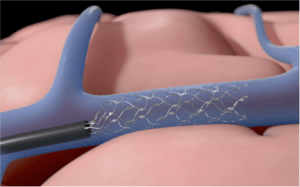
The Stentrode is the only investigational, implantable device that does not require open brain surgery and is designed so that patients can train their brains to wirelessly control external systems without the need for using their hands or voice.
Other neural interface devices, such as those being developed by other companies, including one backed by Elon Musk, currently require drilling into the skull, and direct puncture into the brain, to achieve device implantation.
Safety and efficacy data from a currently-active first-in-human clinical trial will be used to finalise the protocol for a pivotal FDA-enabling study that will guide evaluation for US marketing approval. Future research will evaluate the use of the device in patients with paralysis due to spinal cord injury, amyotrophic lateral sclerosis (ALS), stroke, and muscular dystrophy.
Similar to the procedure utilised for implantation of cardiac pacemakers, implantation of the Stentrode is a minimally-invasive procedure during which the device is delivered to the brain through blood vessels, instead of open brain surgery.
The technology relies on a brain-controlled handsfree app platform called brainOS to translate the brain activity into a standardised digital language, directly through thought, to control apps that restore communication and limb function. In addition, brainPort, a fully internalised, wireless solution implanted in the chest provides high-resolution neural data transmission.
Pre-clinical studies have demonstrated the Stentrode’s long-term safety as well as its ability to pick up specific electrical frequencies emitted by the brain. The company, in collaboration with the University of Melbourne, has published their scientific results in top ranking journals including Nature Biotechnology, Nature Biomedical Engineering, and the Journal of Neurosurgery.









