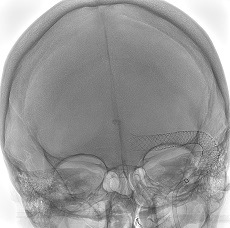
Sonorous NV has announced the treatment of the first patients in North America with the BossStent device, which is designed to treat patients with symptomatic cerebral venous diseases.
As per a Sonorous press release, the single-use, implantable device acts as an endoluminal prosthesis that is placed into a cerebral venous sinus stenosis to normalise blood flow and pressure gradients causing pulsatile tinnitus and/or idiopathic intracranial hypertension (IIH).
The initial cases in North America were performed by Vitor Mendes Pereira (St Michael’s Hospital, Toronto, Canada).
“The BossStent Sonorous NV device produced excellent clinical results,” Pereira said. “It offers a breakthrough solution for patients with symptomatic cerebral venous diseases associated with venous sinus stenosis who require interventional therapy. Knowing such a solution exists places both patients and physicians at ease, and will create opportunities in the future for patients who are experiencing these debilitating symptoms.”
“We are confident that use of the BossStent device will provide an on-label solution for symptomatic cerebral venous diseases leading to papilloedema, pulsatile tinnitus and IIH,” added Sonorous chairman Dave Ferrera. “Over time, we believe use of the device will become the standard of care.”
Sonorous received its initial seed funding in Q1 of 2022 and quickly developed its product in order to achieve first clinical use in less than one year. According to the release, European Union (EU) and US Food and Drug Administration (FDA) clinical trials will be initiated in Q2 2023 for the purpose of obtaining European and US regulatory approvals, respectively, in Q1 2024.







