Sequent Medical has begun enrolling patients in a study which will evaluate the safety and effectiveness of the WEB (woven endobridge) aneurysm embolisation system for the treatment of ruptured intracranial aneurysms.
The first patient was enrolled by Laurent Spelle, head of Neuroradiology, Bicetre University Hospital, Paris, France, who is the study’s principal investigator.
Fifty patients with ruptured aneurysms are to be enrolled in the “Clinical Assessment of WEB device in Ruptured Aneurysms” (CLARYS) study, which will take place at up to 15 sites in France and Germany.
CLARYS will be the first prospective, multicentre study focused only on gathering data on the WEB device in this particular patient population. The primary endpoint of the study will be the rate of aneurysm re-bleeding at 30 days. An independent core lab will review all study data and CLARYS will also feature independent clinical event adjudication.
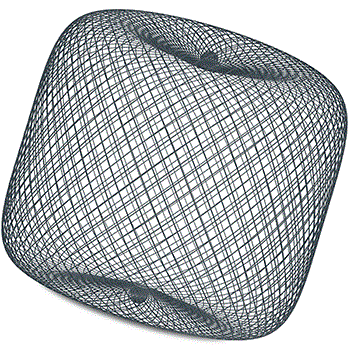
The WEB device consists of a dense mesh constructed from a large number of extremely fine nitinol wires, and functions as an intrasaccular flow disruptor, bridging the neck of the aneurysm and providing rapid, peri-procedural stasis.
“The combination of rapid and durable stasis, a safe, fast procedure and the avoidance of long-term dual antiplatelet therapy makes the WEB an ideal treatment option for ruptured aneurysms,” stated Spelle. “The initiation of the CLARYS study represents the next important milestone for this exciting technology platform, and a critical step towards improving outcomes in a large patient population with significant unmet needs.”









